
EBK THERMODYNAMICS: AN ENGINEERING APPR
8th Edition
ISBN: 8220102809444
Author: CENGEL
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 7.13, Problem 89P
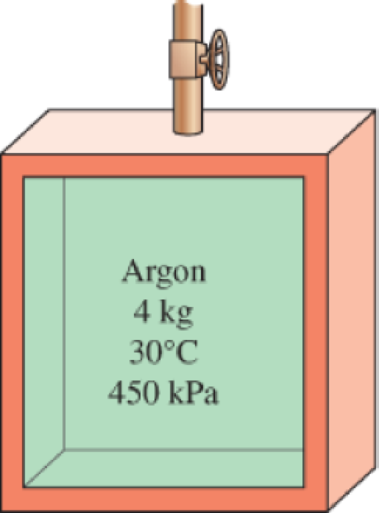
An insulated rigid tank contains 4 kg of argon gas at 450 kPa and 30°C. A valve is now opened, and argon is allowed to escape until the pressure inside drops to 200 kPa. Assuming the argon remaining inside the tank has undergone a reversible, adiabatic process, determine the final mass in the tank.
FIGURE P7–96
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Correct answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you. Expert solution pls
Correct answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you.
Correct answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you.
Chapter 7 Solutions
EBK THERMODYNAMICS: AN ENGINEERING APPR
Ch. 7.13 - Prob. 1PCh. 7.13 - Does the cyclic integral of heat have to be zero...Ch. 7.13 - Is a quantity whose cyclic integral is zero...Ch. 7.13 - Prob. 4PCh. 7.13 - Prob. 5PCh. 7.13 - How do the values of the integral 12Q/T compare...Ch. 7.13 - The entropy of a hot baked potato decreases as it...Ch. 7.13 - When a system is adiabatic, what can be said about...Ch. 7.13 - Prob. 9PCh. 7.13 - A pistoncylinder device contains helium gas....
Ch. 7.13 - A pistoncylinder device contains nitrogen gas....Ch. 7.13 - A pistoncylinder device contains superheated...Ch. 7.13 - The entropy of steam will (increase, decrease,...Ch. 7.13 - Prob. 14PCh. 7.13 - Prob. 15PCh. 7.13 - Prob. 16PCh. 7.13 - Steam is accelerated as it flows through an actual...Ch. 7.13 - Prob. 18PCh. 7.13 - Prob. 19PCh. 7.13 - Prob. 20PCh. 7.13 - Heat in the amount of 100 kJ is transferred...Ch. 7.13 - In Prob. 719, assume that the heat is transferred...Ch. 7.13 - 7–23 A completely reversible heat pump produces...Ch. 7.13 - During the isothermal heat addition process of a...Ch. 7.13 - Prob. 25PCh. 7.13 - During the isothermal heat rejection process of a...Ch. 7.13 - Prob. 27PCh. 7.13 - Prob. 28PCh. 7.13 - Two lbm of water at 300 psia fill a weighted...Ch. 7.13 - A well-insulated rigid tank contains 3 kg of a...Ch. 7.13 - The radiator of a steam heating system has a...Ch. 7.13 - A rigid tank is divided into two equal parts by a...Ch. 7.13 - 7–33 An insulated piston–cylinder device contains...Ch. 7.13 - Prob. 34PCh. 7.13 - Prob. 35PCh. 7.13 - Onekg of R-134a initially at 600 kPa and 25C...Ch. 7.13 - Refrigerant-134a is expanded isentropically from...Ch. 7.13 - Prob. 38PCh. 7.13 - Refrigerant-134a at 320 kPa and 40C undergoes an...Ch. 7.13 - A rigid tank contains 5 kg of saturated vapor...Ch. 7.13 - A 0.5-m3 rigid tank contains refrigerant-134a...Ch. 7.13 - Prob. 44PCh. 7.13 - Prob. 45PCh. 7.13 - Steam enters an adiabatic diffuser at 150 kPa and...Ch. 7.13 - Prob. 47PCh. 7.13 - An isentropic steam turbine processes 2 kg/s of...Ch. 7.13 - Prob. 50PCh. 7.13 -
7–51 0.7-kg of R-134a is expanded isentropically...Ch. 7.13 - Twokg of saturated water vapor at 600 kPa are...Ch. 7.13 - Steam enters a steady-flow adiabatic nozzle with a...Ch. 7.13 - Prob. 54PCh. 7.13 - In Prob. 755, the water is stirred at the same...Ch. 7.13 - A pistoncylinder device contains 5 kg of steam at...Ch. 7.13 - Prob. 57PCh. 7.13 - Prob. 59PCh. 7.13 - A 50-kg copper block initially at 140C is dropped...Ch. 7.13 - Prob. 61PCh. 7.13 - Prob. 62PCh. 7.13 - A 30-kg aluminum block initially at 140C is...Ch. 7.13 - A 30-kg iron block and a 40-kg copper block, both...Ch. 7.13 - An adiabatic pump is to be used to compress...Ch. 7.13 - Prob. 67PCh. 7.13 - Can the entropy of an ideal gas change during an...Ch. 7.13 - An ideal gas undergoes a process between two...Ch. 7.13 - Prob. 72PCh. 7.13 - Prob. 73PCh. 7.13 - Prob. 74PCh. 7.13 - Prob. 75PCh. 7.13 - A 1.5-m3 insulated rigid tank contains 2.7 kg of...Ch. 7.13 - An insulated pistoncylinder device initially...Ch. 7.13 - A pistoncylinder device contains 0.75 kg of...Ch. 7.13 - Prob. 80PCh. 7.13 - 7–81 Air enters a nozzle steadily at 280 kPa and...Ch. 7.13 - A mass of 25 lbm of helium undergoes a process...Ch. 7.13 - One kg of air at 200 kPa and 127C is contained in...Ch. 7.13 - Prob. 85PCh. 7.13 - Air at 3.5 MPa and 500C is expanded in an...Ch. 7.13 -
7–87E Air is compressed in an isentropic...Ch. 7.13 - An insulated rigid tank is divided into two equal...Ch. 7.13 - An insulated rigid tank contains 4 kg of argon gas...Ch. 7.13 - Prob. 90PCh. 7.13 - Prob. 91PCh. 7.13 - Prob. 92PCh. 7.13 - Air at 27C and 100 kPa is contained in a...Ch. 7.13 - Prob. 94PCh. 7.13 - Helium gas is compressed from 90 kPa and 30C to...Ch. 7.13 - Five kg of air at 427C and 600 kPa are contained...Ch. 7.13 - Prob. 97PCh. 7.13 - The well-insulated container shown in Fig. P 795E...Ch. 7.13 - Prob. 99PCh. 7.13 - Prob. 100PCh. 7.13 - It is well known that the power consumed by a...Ch. 7.13 - Prob. 102PCh. 7.13 - Prob. 103PCh. 7.13 - Saturated water vapor at 150C is compressed in a...Ch. 7.13 - Liquid water at 120 kPa enters a 7-kW pump where...Ch. 7.13 - Prob. 106PCh. 7.13 - Consider a steam power plant that operates between...Ch. 7.13 - Helium gas is compressed from 16 psia and 85F to...Ch. 7.13 - Nitrogen gas is compressed from 80 kPa and 27C to...Ch. 7.13 - Saturated refrigerant-134a vapor at 15 psia is...Ch. 7.13 - Describe the ideal process for an (a) adiabatic...Ch. 7.13 - Is the isentropic process a suitable model for...Ch. 7.13 - On a T-s diagram, does the actual exit state...Ch. 7.13 - Steam at 100 psia and 650F is expanded...Ch. 7.13 - Prob. 117PCh. 7.13 - Combustion gases enter an adiabatic gas turbine at...Ch. 7.13 - Steam at 4 MPa and 350C is expanded in an...Ch. 7.13 - Prob. 120PCh. 7.13 - Prob. 122PCh. 7.13 - Prob. 123PCh. 7.13 - Refrigerant-134a enters an adiabatic compressor as...Ch. 7.13 - Prob. 126PCh. 7.13 - Argon gas enters an adiabatic compressor at 14...Ch. 7.13 - Air enters an adiabatic nozzle at 45 psia and 940F...Ch. 7.13 - Prob. 130PCh. 7.13 - An adiabatic diffuser at the inlet of a jet engine...Ch. 7.13 - Hot combustion gases enter the nozzle of a...Ch. 7.13 - Refrigerant-134a is expanded adiabatically from...Ch. 7.13 - Oxygen enters an insulated 12-cm-diameter pipe...Ch. 7.13 - Prob. 135PCh. 7.13 - Prob. 136PCh. 7.13 - Steam enters an adiabatic turbine steadily at 7...Ch. 7.13 - 7–138 In an ice-making plant, water at 0°C is...Ch. 7.13 - Water at 20 psia and 50F enters a mixing chamber...Ch. 7.13 - Prob. 140PCh. 7.13 - Prob. 141PCh. 7.13 - Prob. 142PCh. 7.13 - Prob. 143PCh. 7.13 - In a dairy plant, milk at 4C is pasteurized...Ch. 7.13 - An ordinary egg can be approximated as a...Ch. 7.13 - Prob. 146PCh. 7.13 - Prob. 147PCh. 7.13 - In a production facility, 1.2-in-thick, 2-ft 2-ft...Ch. 7.13 - Prob. 149PCh. 7.13 - Prob. 150PCh. 7.13 - A frictionless pistoncylinder device contains...Ch. 7.13 - Prob. 152PCh. 7.13 - Prob. 153PCh. 7.13 - Prob. 154PCh. 7.13 - Prob. 155PCh. 7.13 - Liquid water at 200 kPa and 15C is heated in a...Ch. 7.13 - Prob. 157PCh. 7.13 - Prob. 158PCh. 7.13 - Prob. 159PCh. 7.13 - Prob. 160PCh. 7.13 - Prob. 161PCh. 7.13 - Prob. 162PCh. 7.13 - Prob. 163PCh. 7.13 - Prob. 164PCh. 7.13 - Prob. 165PCh. 7.13 - The space heating of a facility is accomplished by...Ch. 7.13 - Prob. 167PCh. 7.13 - Prob. 168PCh. 7.13 - Prob. 169RPCh. 7.13 - A refrigerator with a coefficient of performance...Ch. 7.13 - Prob. 171RPCh. 7.13 - Prob. 172RPCh. 7.13 - Prob. 173RPCh. 7.13 - A 100-lbm block of a solid material whose specific...Ch. 7.13 - Prob. 175RPCh. 7.13 - Prob. 176RPCh. 7.13 - A pistoncylinder device initially contains 15 ft3...Ch. 7.13 - Prob. 178RPCh. 7.13 - A 0.8-m3 rigid tank contains carbon dioxide (CO2)...Ch. 7.13 - Helium gas is throttled steadily from 400 kPa and...Ch. 7.13 - Air enters the evaporator section of a window air...Ch. 7.13 - Refrigerant-134a enters a compressor as a...Ch. 7.13 - Prob. 183RPCh. 7.13 - Three kg of helium gas at 100 kPa and 27C are...Ch. 7.13 - Prob. 185RPCh. 7.13 -
7–186 You are to expand a gas adiabatically from...Ch. 7.13 - Prob. 187RPCh. 7.13 - Determine the work input and entropy generation...Ch. 7.13 - Prob. 189RPCh. 7.13 - Prob. 190RPCh. 7.13 - Air enters a two-stage compressor at 100 kPa and...Ch. 7.13 - Steam at 6 MPa and 500C enters a two-stage...Ch. 7.13 - Prob. 193RPCh. 7.13 - Prob. 194RPCh. 7.13 - Prob. 196RPCh. 7.13 - Prob. 197RPCh. 7.13 - 7–198 To control the power output of an isentropic...Ch. 7.13 - Prob. 199RPCh. 7.13 - Prob. 200RPCh. 7.13 - A 5-ft3 rigid tank initially contains...Ch. 7.13 - Prob. 202RPCh. 7.13 - Prob. 203RPCh. 7.13 - Prob. 204RPCh. 7.13 - Prob. 205RPCh. 7.13 - Prob. 206RPCh. 7.13 - Prob. 207RPCh. 7.13 - Prob. 208RPCh. 7.13 - (a) Water flows through a shower head steadily at...Ch. 7.13 - Prob. 211RPCh. 7.13 - Prob. 212RPCh. 7.13 - Prob. 213RPCh. 7.13 - Consider the turbocharger of an internal...Ch. 7.13 - Prob. 215RPCh. 7.13 - Prob. 216RPCh. 7.13 - Prob. 217RPCh. 7.13 - Consider two bodies of identical mass m and...Ch. 7.13 - Prob. 220RPCh. 7.13 - Prob. 222RPCh. 7.13 - Prob. 224RPCh. 7.13 - The polytropic or small stage efficiency of a...Ch. 7.13 - Steam is compressed from 6 MPa and 300C to 10 MPa...Ch. 7.13 - An apple with a mass of 0.12 kg and average...Ch. 7.13 - A pistoncylinder device contains 5 kg of saturated...Ch. 7.13 - Prob. 229FEPCh. 7.13 - Prob. 230FEPCh. 7.13 - A unit mass of a substance undergoes an...Ch. 7.13 - A unit mass of an ideal gas at temperature T...Ch. 7.13 - Prob. 233FEPCh. 7.13 - Prob. 234FEPCh. 7.13 - Air is compressed steadily and adiabatically from...Ch. 7.13 - Argon gas expands in an adiabatic turbine steadily...Ch. 7.13 - Water enters a pump steadily at 100 kPa at a rate...Ch. 7.13 - Air is to be compressed steadily and...Ch. 7.13 - Helium gas enters an adiabatic nozzle steadily at...Ch. 7.13 - Combustion gases with a specific heat ratio of 1.3...Ch. 7.13 - Steam enters an adiabatic turbine steadily at 400C...Ch. 7.13 - Liquid water enters an adiabatic piping system at...Ch. 7.13 - Prob. 243FEPCh. 7.13 - Steam enters an adiabatic turbine at 8 MPa and...Ch. 7.13 - Helium gas is compressed steadily from 90 kPa and...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Correct answer is written below. Detailed and complete solution only with fbd. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you.arrow_forward
- Correct answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forward
- Correct answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you. Prefferably handwritten solution pleasearrow_forward
- Correct answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you. Prefferably handwritten solution pleasearrow_forwardCorrect answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you. Prefferably handwritten solution pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Power Plant Explained | Working Principles; Author: RealPars;https://www.youtube.com/watch?v=HGVDu1z5YQ8;License: Standard YouTube License, CC-BY