
THERMODYNAMICS (LL)-W/ACCESS >CUSTOM<
9th Edition
ISBN: 9781266657610
Author: CENGEL
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7.13, Problem 221RP
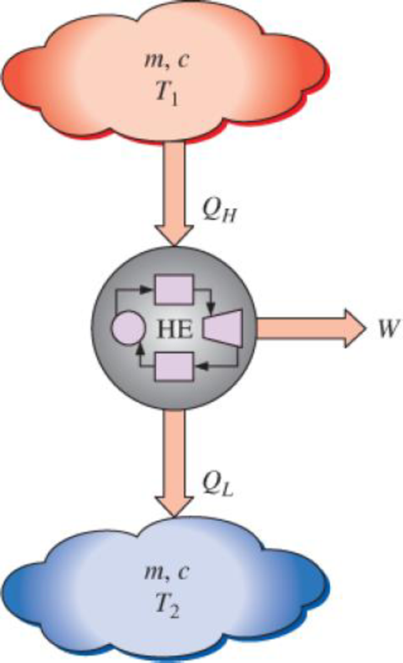
Consider two bodies of identical mass m and specific heat c used as thermal reservoirs (source and sink) for a heat engine. The first body is initially at an absolute temperature T1 while the second one is at a lower absolute temperature T2. Heat is transferred from the first body to the heat engine, which rejects the waste heat to the second body. The process continues until the final temperatures of the two bodies Tf become equal. Show that
FIGURE P7–221
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please do not rely too much on chatgpt, because its answer may be wrong. Please consider it carefully and give your own answer. You can borrow ideas from gpt, but please do not believe its answer.Very very grateful!
Please do not copy other's work,i will be very very grateful!!Please do not copy other's work,i will be very very grateful!!
=
The frame shown is fitted with three 50 cm diameter
frictionless pulleys. A force of F = 630 N is applied to the
rope at an angle ◊ 43°. Member ABCD is attached to the
wall by a fixed support at A. Find the forces indicated below.
Note: The rope is tangent to the pully (D) and not secured at
the 3 o'clock position.
a
b
•C
*су
G
E
e
d
BY NC SA
2013 Michael Swanbom
Values for dimensions on the figure are given in the following
table. Note the figure may not be to scale.
Variable Value
a
81 cm
b
50 cm
с
59 cm
d
155 cm
For all answers, take x as positive to the right and
positive upward.
At point A, the fixed support exerts a force of:
A
=
+
ĴN
and a reaction couple of:
→>
ΜΑ
Member CG is in Select an answer
magnitude
У
as
k N-m.
and carries a force of
N.
The lower jaw AB [Purple 1] and the upper jaw-handle AD
[Yellow 2] exert vertical clamping forces on the object at R.
The hand squeezes the upper jaw-handle AD [2] and the
lower handle BC [Orane 4] with forces F. (Member CD [Red 3]
acts as if it is pinned at D, but, in a real vise-grips, its
position is actually adjustable.) The clamping force, R,
depends on the geometry and on the squeezing force F
applied to the handles. Determine the proportionality
between the clamping force, R, and the squeezing force F for
the dimensions given.
d3
d4
R
1
B
d1
2
d2
D...
d5
F
4
F
Values for dimensions on the figure are given in the following
table. Note the figure may not be to scale.
Variable
Value
d1
65 mm
d2
156 mm
d3
50 mm
45
d4
d5
113 mm
30 mm
R =
F
Chapter 7 Solutions
THERMODYNAMICS (LL)-W/ACCESS >CUSTOM<
Ch. 7.13 - Does a cycle for which Q 0 violate the Clausius...Ch. 7.13 - Does the cyclic integral of heat have to be zero...Ch. 7.13 - Is a quantity whose cyclic integral is zero...Ch. 7.13 - Prob. 4PCh. 7.13 - Prob. 5PCh. 7.13 - How do the values of the integral 12Q/T compare...Ch. 7.13 - Prob. 7PCh. 7.13 - The entropy of a hot baked potato decreases as it...Ch. 7.13 - When a system is adiabatic, what can be said about...Ch. 7.13 - Prob. 10P
Ch. 7.13 - A pistoncylinder device contains helium gas....Ch. 7.13 - A pistoncylinder device contains nitrogen gas....Ch. 7.13 - A pistoncylinder device contains superheated...Ch. 7.13 - The entropy of steam will (increase, decrease,...Ch. 7.13 - During a heat transfer process, the entropy of a...Ch. 7.13 - Steam is accelerated as it flows through an actual...Ch. 7.13 - Heat is transferred at a rate of 2 kW from a hot...Ch. 7.13 - A completely reversible air conditioner provides...Ch. 7.13 - Heat in the amount of 100 kJ is transferred...Ch. 7.13 - In Prob. 719, assume that the heat is transferred...Ch. 7.13 - During the isothermal heat addition process of a...Ch. 7.13 - Prob. 22PCh. 7.13 - During the isothermal heat rejection process of a...Ch. 7.13 - Air is compressed by a 40-kW compressor from P1 to...Ch. 7.13 - Refrigerant-134a enters the coils of the...Ch. 7.13 - A rigid tank contains an ideal gas at 40C that is...Ch. 7.13 - A rigid vessel is filled with a fluid from a...Ch. 7.13 - A rigid vessel filled with a fluid is allowed to...Ch. 7.13 - Prob. 29PCh. 7.13 - One lbm of R-134a is expanded isentropically in a...Ch. 7.13 - Two lbm of water at 300 psia fill a weighted...Ch. 7.13 - A well-insulated rigid tank contains 3 kg of a...Ch. 7.13 - Using the relation ds = (Q/T)int rev for the...Ch. 7.13 - The radiator of a steam heating system has a...Ch. 7.13 - A rigid tank is divided into two equal parts by a...Ch. 7.13 - Prob. 36PCh. 7.13 - An insulated pistoncylinder device contains 5 L of...Ch. 7.13 - Onekg of R-134a initially at 600 kPa and 25C...Ch. 7.13 - Refrigerant-134a is expanded isentropically from...Ch. 7.13 - Refrigerant-134a at 320 kPa and 40C undergoes an...Ch. 7.13 - A rigid tank contains 5 kg of saturated vapor...Ch. 7.13 - A 0.5-m3 rigid tank contains refrigerant-134a...Ch. 7.13 - Steam enters a steady-flow adiabatic nozzle with a...Ch. 7.13 - Steam enters an adiabatic diffuser at 150 kPa and...Ch. 7.13 - R-134a vapor enters into a turbine at 250 psia and...Ch. 7.13 - Refrigerant-134a enters an adiabatic compressor as...Ch. 7.13 - The compressor in a refrigerator compresses...Ch. 7.13 - An isentropic steam turbine processes 2 kg/s of...Ch. 7.13 - Prob. 52PCh. 7.13 - Twokg of saturated water vapor at 600 kPa are...Ch. 7.13 - A pistoncylinder device contains 5 kg of steam at...Ch. 7.13 - Prob. 55PCh. 7.13 - In Prob. 755, the water is stirred at the same...Ch. 7.13 - Prob. 57PCh. 7.13 - Prob. 58PCh. 7.13 - Determine the total heat transfer for the...Ch. 7.13 - Calculate the heat transfer, in kJ/kg. for the...Ch. 7.13 - Prob. 61PCh. 7.13 - An adiabatic pump is to be used to compress...Ch. 7.13 - Prob. 63PCh. 7.13 - Prob. 64PCh. 7.13 - A 30-kg aluminum block initially at 140C is...Ch. 7.13 - A 50-kg copper block initially at 140C is dropped...Ch. 7.13 - A 30-kg iron block and a 40-kg copper block, both...Ch. 7.13 - Prob. 69PCh. 7.13 - Prob. 70PCh. 7.13 - Can the entropy of an ideal gas change during an...Ch. 7.13 - An ideal gas undergoes a process between two...Ch. 7.13 - Prob. 73PCh. 7.13 - Air is expanded from 200 psia and 500F to 100 psia...Ch. 7.13 - Prob. 75PCh. 7.13 - Air is expanded isentropically from 100 psia and...Ch. 7.13 - Which of the two gaseshelium or nitrogenhas the...Ch. 7.13 - Which of the two gasesneon or airhas the lower...Ch. 7.13 - A 1.5-m3 insulated rigid tank contains 2.7 kg of...Ch. 7.13 - An insulated pistoncylinder device initially...Ch. 7.13 - A pistoncylinder device contains 0.75 kg of...Ch. 7.13 - A mass of 25 lbm of helium undergoes a process...Ch. 7.13 - One kg of air at 200 kPa and 127C is contained in...Ch. 7.13 - An insulated rigid tank is divided into two equal...Ch. 7.13 - Air at 27C and 100 kPa is contained in a...Ch. 7.13 - Air at 3.5 MPa and 500C is expanded in an...Ch. 7.13 - Air is compressed in a pistoncylinder device from...Ch. 7.13 - Helium gas is compressed from 90 kPa and 30C to...Ch. 7.13 - Nitrogen at 120 kPa and 30C is compressed to 600...Ch. 7.13 - Five kg of air at 427C and 600 kPa are contained...Ch. 7.13 - Prob. 92PCh. 7.13 - Prob. 93PCh. 7.13 - Prob. 94PCh. 7.13 - The well-insulated container shown in Fig. P 795E...Ch. 7.13 - An insulated rigid tank contains 4 kg of argon gas...Ch. 7.13 - Prob. 97PCh. 7.13 - Prob. 98PCh. 7.13 - Prob. 99PCh. 7.13 - It is well known that the power consumed by a...Ch. 7.13 - Calculate the work produced, in kJ/kg, for the...Ch. 7.13 - Prob. 102PCh. 7.13 - Prob. 103PCh. 7.13 - Saturated water vapor at 150C is compressed in a...Ch. 7.13 - Liquid water at 120 kPa enters a 7-kW pump where...Ch. 7.13 - Water enters the pump of a steam power plant as...Ch. 7.13 - Consider a steam power plant that operates between...Ch. 7.13 - Saturated refrigerant-134a vapor at 15 psia is...Ch. 7.13 - Helium gas is compressed from 16 psia and 85F to...Ch. 7.13 - Nitrogen gas is compressed from 80 kPa and 27C to...Ch. 7.13 - Describe the ideal process for an (a) adiabatic...Ch. 7.13 - Is the isentropic process a suitable model for...Ch. 7.13 - On a T-s diagram, does the actual exit state...Ch. 7.13 - Argon gas enters an adiabatic turbine at 800C and...Ch. 7.13 - Steam at 100 psia and 650F is expanded...Ch. 7.13 - Combustion gases enter an adiabatic gas turbine at...Ch. 7.13 - Steam at 4 MPa and 350C is expanded in an...Ch. 7.13 - Prob. 120PCh. 7.13 - Prob. 121PCh. 7.13 - Refrigerant-134a enters an adiabatic compressor as...Ch. 7.13 - The adiabatic compressor of a refrigeration system...Ch. 7.13 - Prob. 125PCh. 7.13 - Argon gas enters an adiabatic compressor at 14...Ch. 7.13 - Prob. 127PCh. 7.13 - Air enters an adiabatic nozzle at 45 psia and 940F...Ch. 7.13 - An adiabatic diffuser at the inlet of a jet engine...Ch. 7.13 - Hot combustion gases enter the nozzle of a...Ch. 7.13 - The exhaust nozzle of a jet engine expands air at...Ch. 7.13 - Prob. 133PCh. 7.13 - Refrigerant-134a is expanded adiabatically from...Ch. 7.13 - A frictionless pistoncylinder device contains...Ch. 7.13 - Prob. 136PCh. 7.13 - Steam enters an adiabatic turbine steadily at 7...Ch. 7.13 - Prob. 138PCh. 7.13 - Oxygen enters an insulated 12-cm-diameter pipe...Ch. 7.13 - Water at 20 psia and 50F enters a mixing chamber...Ch. 7.13 - Prob. 141PCh. 7.13 - Prob. 142PCh. 7.13 - In a dairy plant, milk at 4C is pasteurized...Ch. 7.13 - Steam is to be condensed in the condenser of a...Ch. 7.13 - An ordinary egg can be approximated as a...Ch. 7.13 - Prob. 146PCh. 7.13 - In a production facility, 1.2-in-thick, 2-ft 2-ft...Ch. 7.13 - Prob. 148PCh. 7.13 - Prob. 149PCh. 7.13 - Prob. 150PCh. 7.13 - Prob. 151PCh. 7.13 - Prob. 152PCh. 7.13 - Prob. 153PCh. 7.13 - Liquid water at 200 kPa and 15C is heated in a...Ch. 7.13 - Prob. 155PCh. 7.13 - Prob. 157PCh. 7.13 - Prob. 158PCh. 7.13 - Prob. 159PCh. 7.13 - Prob. 160PCh. 7.13 - The compressed-air requirements of a plant are met...Ch. 7.13 - Prob. 162PCh. 7.13 - The space heating of a facility is accomplished by...Ch. 7.13 - Prob. 164PCh. 7.13 - Prob. 165PCh. 7.13 - Prob. 166PCh. 7.13 - Prob. 167RPCh. 7.13 - A refrigerator with a coefficient of performance...Ch. 7.13 - What is the minimum internal energy that steam can...Ch. 7.13 - Prob. 170RPCh. 7.13 - What is the maximum volume that 3 kg of oxygen at...Ch. 7.13 - A 100-lbm block of a solid material whose specific...Ch. 7.13 - Prob. 173RPCh. 7.13 - A pistoncylinder device initially contains 15 ft3...Ch. 7.13 - A pistoncylinder device contains steam that...Ch. 7.13 - Prob. 176RPCh. 7.13 - Prob. 177RPCh. 7.13 - Prob. 178RPCh. 7.13 - A 0.8-m3 rigid tank contains carbon dioxide (CO2)...Ch. 7.13 - Air enters the evaporator section of a window air...Ch. 7.13 - Prob. 181RPCh. 7.13 - Prob. 182RPCh. 7.13 - Prob. 183RPCh. 7.13 - Prob. 184RPCh. 7.13 - Helium gas is throttled steadily from 400 kPa and...Ch. 7.13 - Determine the work input and entropy generation...Ch. 7.13 - Prob. 187RPCh. 7.13 - Reconsider Prob. 7187. Determine the change in the...Ch. 7.13 - Prob. 189RPCh. 7.13 - Air enters a two-stage compressor at 100 kPa and...Ch. 7.13 - Three kg of helium gas at 100 kPa and 27C are...Ch. 7.13 - Steam at 6 MPa and 500C enters a two-stage...Ch. 7.13 - Prob. 193RPCh. 7.13 - Prob. 194RPCh. 7.13 - Refrigerant-134a enters a compressor as a...Ch. 7.13 - Prob. 196RPCh. 7.13 - Prob. 197RPCh. 7.13 - Prob. 198RPCh. 7.13 - Prob. 199RPCh. 7.13 - Prob. 200RPCh. 7.13 - Prob. 201RPCh. 7.13 - Prob. 202RPCh. 7.13 - Prob. 203RPCh. 7.13 - Prob. 204RPCh. 7.13 - Prob. 205RPCh. 7.13 - Prob. 206RPCh. 7.13 - Prob. 207RPCh. 7.13 - Prob. 208RPCh. 7.13 - (a) Water flows through a shower head steadily at...Ch. 7.13 - Prob. 211RPCh. 7.13 - Prob. 212RPCh. 7.13 - Prob. 213RPCh. 7.13 - Consider the turbocharger of an internal...Ch. 7.13 - Prob. 215RPCh. 7.13 - Prob. 216RPCh. 7.13 - A 5-ft3 rigid tank initially contains...Ch. 7.13 - Prob. 218RPCh. 7.13 - Show that the difference between the reversible...Ch. 7.13 - Demonstrate the validity of the Clausius...Ch. 7.13 - Consider two bodies of identical mass m and...Ch. 7.13 - Consider a three-stage isentropic compressor with...Ch. 7.13 - Prob. 223RPCh. 7.13 - Prob. 224RPCh. 7.13 - Prob. 225RPCh. 7.13 - The polytropic or small stage efficiency of a...Ch. 7.13 - Steam is condensed at a constant temperature of...Ch. 7.13 - Steam is compressed from 6 MPa and 300C to 10 MPa...Ch. 7.13 - An apple with a mass of 0.12 kg and average...Ch. 7.13 - A pistoncylinder device contains 5 kg of saturated...Ch. 7.13 - Argon gas expands in an adiabatic turbine from 3...Ch. 7.13 - A unit mass of a substance undergoes an...Ch. 7.13 - A unit mass of an ideal gas at temperature T...Ch. 7.13 - Heat is lost through a plane wall steadily at a...Ch. 7.13 - Air is compressed steadily and adiabatically from...Ch. 7.13 - Argon gas expands in an adiabatic turbine steadily...Ch. 7.13 - Water enters a pump steadily at 100 kPa at a rate...Ch. 7.13 - Air is to be compressed steadily and...Ch. 7.13 - Helium gas enters an adiabatic nozzle steadily at...Ch. 7.13 - Combustion gases with a specific heat ratio of 1.3...Ch. 7.13 - Steam enters an adiabatic turbine steadily at 400C...Ch. 7.13 - Liquid water enters an adiabatic piping system at...Ch. 7.13 - Liquid water is to be compressed by a pump whose...Ch. 7.13 - Steam enters an adiabatic turbine at 8 MPa and...Ch. 7.13 - Helium gas is compressed steadily from 90 kPa and...Ch. 7.13 - Helium gas is compressed from 1 atm and 25C to a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- A triangular distributed load of max intensity w =460 N/m acts on beam AB. The beam is supported by a pin at A and member CD, which is connected by pins at C and D respectively. Determine the reaction forces at A and C. Enter your answers in Cartesian components. Assume the masses of both beam AB and member CD are negligible. cc 040 BY NC SA 2016 Eric Davishahl W A C D -a- B Ул -b- x Values for dimensions on the figure are given in the following table. Note the figure may not be to scale. Variable Value α 5.4 m b 8.64 m C 3.24 m The reaction at A is A = i+ ĴN. λ = i+ Ĵ N. The reaction at C is C =arrow_forward56 Clamps like the one shown are commonly used in woodworking applications. This clamp has the dimensions given in the table below the figure, and its jaws are mm thick (in the direction perpendicular to the plane of the picture). a.) The screws of the clamp are adjusted so that there is a uniform pressure of P = 150 kPa being applied to the workpieces by the jaws. Determine the force carried in each screw. Hint: the uniform pressure can be modeled in 2-D as a uniform distributed load with intensity w = Pt (units of N/m) acting over the length of contact between the jaw and the workpiece. b.) Determine the minimum vertical force (parallel to the jaws) required to pull either one of the workpieces out of the clamp jaws. Use a coefficient of static friction between all contacting surfaces of μs = 0.56 and the same clamping pressure given for part (a). 2013 Michael Swanbom A B C a Values for dimensions on the figure are given in the following table. Note the figure may not be to scale.…arrow_forwardDetermine the force in each member of the space truss given F=5 kN. Use positive to indicate tension and negative to indicate compression. F E Z -2 m. B 3 m C 5 m 3 m A -4 m. AB = KN FAC = FAD = KN KN KN FBC = KN FBD FBE = = KN Farrow_forward
- A short brass cyclinder (denisty=8530 kg/m^3, cp=0.389 kJ/kgK, k=110 W/mK, and alpha=3.39*10^-5 m^2/s) of diameter 4 cm and height 20 cm is initially at uniform temperature of 150 degrees C. The cylinder is now placed in atmospheric air at 20 degrees C, where heat transfer takes place by convection with a heat transfer coefficent of 40 W/m^2K. Calculate (a) the center temp of the cylinder, (b) the center temp of the top surface of the cylinder, and (c) the total heat transfer from the cylinder 15 min after the start of the cooling. Solve this problem using the analytical one term approximation method. (Answer: (a) 45.7C, (b)45.3C, (c)87.2 kJ)arrow_forwardA short brass cyclinder (denisty=8530 kg/m^3, cp=0.389 kJ/kgK, k=110 W/mK, and alpha=3.39*10^-5 m^2/s) of diameter 4 cm and height 20 cm is initially at uniform temperature of 150 degrees C. The cylinder is now placed in atmospheric air at 20 degrees C, where heat transfer takes place by convection with a heat transfer coefficent of 40 W/m^2K. Calculate (a) the center temp of the cylinder, (b) the center temp of the top surface of the cylinder, and (c) the total heat transfer from the cylinder 15 min after the start of the cooling. Solve this problem using the analytical one term approximation method.arrow_forwardA 6 cm high rectangular ice block (k=2.22 W/mK, and alpha=0.124*10^-7 m^2/s) initially at -18 degrees C is placed on a table on its square base 4 cm by 4cm in size in a room at 18 degrees C. The heat transfer coefficent on the exposed surfaces of the ice block is 12 W/m^2K. Disregarding any heat transfer from the base to the table, determine how long it will be before the ice block starts melting. Where on the ice block will the first liquid droplets appear? Solve this problem using the analytical one-term approximation method.arrow_forward
- Consider a piece of steel undergoing a decarburization process at 925 degrees C. the mass diffusivity of carbon in steel at 925 degrees C is 1*10^-7 cm^2/s. Determine the depth below the surface of the steel at which the concentration of carbon is reduced to 40 percent from its initial value as a result of the decarburization process for (a) an hour and (b) 10 hours. Assume the concnetration of carbon at the surface is zero throughout the decarburization process.arrow_forwardPlease do not rely too much on chatgpt, because its answer may be wrong. Please consider it carefully and give your own answer. You can borrow ideas from gpt, but please do not believe its answer.Very very grateful! Please do not copy other's work,i will be very very grateful!!arrow_forwardMultiple Choice Circle the best answer to each statement. 1. Which geometry attribute deviation(s) can be limited with a profile of a surface tolerance? A. Location B. Orientation C. Form D. All of the above 2. A true profile may be defined with: A. Basic radii B. Basic angles C. Formulas D. All of the above 3. Which modifier may be applied to the profile tolerance value? A B C. D. All of the above 4. The default tolerance zone for a profile tolerance is: A. Non-uniform B. Unilateral C. Bilateral equal distribution D. Bilateral-unequal distribution 5. An advantage of using a profile tolerance in place of a coordinate tolerance is: A. A bonus tolerance is permitted. B. A datum feature sequence may be specified C. A profile tolerance always controls size D. All of the above 6. The shape of the tolerance zone for a profile tolerance is: A. Two parallel planes B. The same as the true profile of the toleranced surface C. Equal bilateral D. Cylindrical when the diameter symbol is speci- fied…arrow_forward
- One thousand kg/h of a (50-50 wt%) acetone-in-water solution is to be extracted at 25C in a continuous, countercurrent system with pure 1,1,2-trichloroethane to obtain a raffinate containing 10 wt% acetone. Using the following equilibrium data, determine with an equilateral-triangle diagram: a- the minimum flow rate of solvent; b- the number of stages required for a solvent rate equal to 1.5 times minimum, and composition of each streamleaving each stage. c- Repeat the calculation of (a) and (b) if the solvent used has purity 93wt% (4wr% acetone, 3wt% water impurities) acetone water 1,1,2-trichloroethane Raffinate. Weight Extract. Weight 0.6 0.13 0.27 Fraction Acetone Fraction Acetone 0.5 0.04 0.46 0.44 0.56 0.4 0.03 0.57 0.29 0.40 0.3 0.02 0.68 0.12 0.18 0.2 0.015 0.785 0.0 0.0 0.1 0.01 0.89 0.55 0.35 0.1 0.5 0.43 0.07 0.4 0.57 0.03 0.3 0.68 0.02 0.2 0.79 0.01 0.1 0.895 0.005arrow_forward2500 kg/hr of (20-80) nicotine water solution is to be extracted with benzene containing 0.5% nicotine in the 1st and 2ed stages while the 3rd stage is free of nicotine. Cross- current operation is used with different amounts of solvent for each stages 2000kg/hr in the 1st stage, 2300 kg/hr in the 2nd stage, 2600 kg/hr in the 3rd, determine: - a- The final raffinate concentration and % extraction. b- b- The minimum amount of solvent required for counter-current operation if the minimum concentration will be reduced to 5% in the outlet raffinate. Equilibrium data Wt % Nicotine in water Wt % Nicotine in benzene 0 4 16 25 0 4 21 30arrow_forwardQuiz/An eccentrically loaded bracket is welded to the support as shown in Figure below. The load is static. The weld size for weld w1 is h1=6mm, for w2 h2 5mm, and for w3 is h3 -5.5 mm. Determine the safety factor (S.f) for the welds. F=22 kN. Use an AWS Electrode type (E90xx). 140 101.15 REDMI NOTE 8 PRO AI QUAD CAMERA Farrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=NyOYW07-L5g;License: Standard youtube license