
EP INTRO.TO GENERAL,ORGANIC...-OWL ACCE
12th Edition
ISBN: 9781337915984
Author: Bettelheim
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 7.1, Problem 7.1QC
Problem 7-1
In the reaction
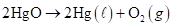
we measure the evolution of oxygen gas to determine the
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which of the following are descriptions of possible starting material for this
reaction?
H
?
trace acid
an ester
a ketone
an imine
an aldehyde
a carboxylic acid
an enamine
a primary amine
a secondary amine
a tertiary amine
None
What are the reagents needed for this and the third structure I only got the top right structure right
Chapter 7 Solutions
EP INTRO.TO GENERAL,ORGANIC...-OWL ACCE
Ch. 7.1 - Problem 7-1 In the reaction we measure the...Ch. 7.4 - Problem 7-2 Calculate the rate for the reaction in...Ch. 7.6 - Prob. 7.3QCCh. 7.6 - Prob. 7.4QCCh. 7.6 - Prob. 7.5QCCh. 7.7 - Prob. 7.6QCCh. 7.7 - Problem 7-7 Consider the following equilibrium...Ch. 7.7 - Prob. 7.8QCCh. 7.7 - Prob. 7.9QCCh. 7 - 7-11 Consider the following reaction: Suppose we...
Ch. 7 - 7-12 Two kinds of gas molecules are reacted at a...Ch. 7 - 7-13 Why are reactions between ions in aqueous...Ch. 7 - Prob. 4PCh. 7 - 7-15 A certain reaction is exothermic by 9...Ch. 7 - 7-16 A quart of milk quickly spoils if left at...Ch. 7 - 7-17 If a certain reaction takes 16 h to go to...Ch. 7 - Prob. 8PCh. 7 - Prob. 9PCh. 7 - Prob. 10PCh. 7 - Prob. 11PCh. 7 - 7-22 If you add a piece of marble, CaCO3 to a 6 M...Ch. 7 - Prob. 13PCh. 7 - Prob. 14PCh. 7 - Prob. 15PCh. 7 - 7-26 Write the chemical equations corresponding to...Ch. 7 - Prob. 17PCh. 7 - 7-28 When the following reaction reached...Ch. 7 - 7-29 The following reaction was allowed to reach...Ch. 7 - Prob. 20PCh. 7 - 7-31 Here are equilibrium constants for several...Ch. 7 - 7-32 A particular reaction has an equilibrium...Ch. 7 - Prob. 23PCh. 7 - Prob. 24PCh. 7 - 7-35 A reaction has a high rate constant but a...Ch. 7 - 7-36 Complete the following table showing the...Ch. 7 - Prob. 27PCh. 7 - Prob. 28PCh. 7 - Prob. 29PCh. 7 - 7-40 Is there any change in conditions that change...Ch. 7 - 7-41 The equilibrium constant at 1127°C for the...Ch. 7 - Prob. 32PCh. 7 - 7-43 (Chemical Connections 7A and 7B) Why is a...Ch. 7 - Prob. 34PCh. 7 - 7-45 (Chemical Connections 7C) A painkiller—for...Ch. 7 - 7-46 (Chemical Connections 7D) What reaction takes...Ch. 7 - Prob. 37PCh. 7 - Prob. 38PCh. 7 - Prob. 39PCh. 7 - 7-50 Draw an energy diagram for an exothermic...Ch. 7 - Prob. 41PCh. 7 - Prob. 42PCh. 7 - Prob. 43PCh. 7 - Prob. 44PCh. 7 - Prob. 45PCh. 7 - Prob. 46PCh. 7 - 7-57 Write the reaction to which the following...Ch. 7 - Prob. 48PCh. 7 - Prob. 49PCh. 7 - Prob. 50PCh. 7 - Prob. 51PCh. 7 - Prob. 52PCh. 7 - Prob. 53PCh. 7 - Prob. 54PCh. 7 - Prob. 55PCh. 7 - Prob. 56PCh. 7 - Prob. 57PCh. 7 - Prob. 58PCh. 7 - 7-69 Pure carbon exists is several forms, two of...Ch. 7 - Prob. 60PCh. 7 - 7-71 You have a beaker that contains solid silver...Ch. 7 - Prob. 62PCh. 7 - Prob. 63PCh. 7 - Prob. 64PCh. 7 - Prob. 65PCh. 7 - Prob. 66PCh. 7 - Prob. 67PCh. 7 - Prob. 68PCh. 7 - Prob. 69PCh. 7 - Prob. 70PCh. 7 - Prob. 71PCh. 7 - Prob. 72PCh. 7 - Prob. 73PCh. 7 - Prob. 74PCh. 7 - 7-82 An equilibrium mixture of O2, SO2, and SO3...Ch. 7 - Prob. 76PCh. 7 - Prob. 77P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please label this COZY spectraarrow_forwardPlease label this HNMRarrow_forwardConsider the following gas chromatographs of Compound A, Compound B, and a mixture of Compounds A and B. Inject A B mixture Area= 9 Area = 5 Area = 3 Area Inject . མི། Inject J2 What is the percentage of Compound B in the the mixture?arrow_forward
- Rank these according to stability. CH3 H3C CH3 1 CH3 H3C 1 most stable, 3 least stable O 1 most stable, 2 least stable 2 most stable, 1 least stable O2 most stable, 3 least stable O3 most stable, 2 least stable O3 most stable, 1 least stable CH3 2 CH3 CH3 H₂C CH3 3 CH3 CHarrow_forwardConsider this IR and NMR: INFRARED SPECTRUM TRANSMITTANCE 0.8- 0.6 0.4 0.2 3000 10 9 8 00 HSP-00-541 7 CO 6 2000 Wavenumber (cm-1) сл 5 ppm 4 M Which compound gave rise to these spectra? N 1000 1 0arrow_forwardConsider this reaction (molecular weights are under each compound): HC=CH + 2 HCI --> C2H4Cl 2 MW = 26 36.5 99 If 4.4 g of HC=CH are reacted with 110 mL of a 2.3 M HCI solution, and 6.0 g of product are actually produced, what is the percent yield?arrow_forward
- What is the name of the major product of this reaction? OH CH3 H₂SO4, heat 1-methylcyclohexene O2-methyl-1-cyclohexene O 3-mthylcyclohexene 1-methyl-2-cyclohexenearrow_forwardWe added a brown solution of Br2 to one of our products, and the brown color disappeared. This indicated that our product wasarrow_forwardRank the following according to reactivity toward nitration: a) benzene b) bromobenzene c) nitrobenzene d) phenol Od) greatest, c) least Od) greatest, b) least Od) greatest, a) least a) greatest, b) least a) greatest, c) least Oa) greatest, d) least Ob) greatest, a) least O b) greatest, c) least Ob) greatest, d) least O c) greatest, a) least O c) greatest, b) least O c) greatest, d) leastarrow_forward
- O-Nitrophenol was distilled over with the steam in our experiment while the other isomer did not. This is due to: O intramolecular hydrogen bonding in the ortho isomer O intermolecular hydrogen bonding in the the ortho isomer O the ortho isomer has a lower density O the ortho isomer has a lower molecular weightarrow_forwardK 44% Problem 68 of 15 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. :6: :: :CI: CI CI: :0:0 Select to Add Arrows Select to Add Arrows H H Cl CI: CI CI: Select to Add Arrows Select to Add Arrows H :CI: Alarrow_forwardI I H :0: Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. 0:0 :0: CI ΑΙ :CI: :CI: :0: CI Select to Add Arrows Select to Add Arrows cl. :0: Cl © ハ CI:: CI H CO Select to Add Arrows Select to Add Arrows 10: AI ::arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32; Author: Crash Course;https://www.youtube.com/watch?v=7qOFtL3VEBc;License: Standard YouTube License, CC-BY