
Chemistry: Atoms First
18th Edition
ISBN: 9781938168154
Author: Richard Langley, Klaus Theopold, Paul Flowers
Publisher: OpenStax College
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 7, Problem 8E
Fill in the blank with a single chemical formula for a covalent compound that will balance the equation:
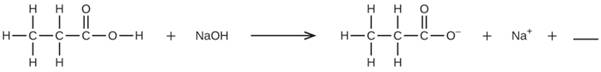
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Please draw, not just describe!
can you draw each step on a piece of a paper please this is very confusing to me
>
Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating
the reactants?
esc
?
A
O
O
•If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like.
• If your answer is no, check the box under the drawing area instead.
olo
18
Ar
Explanation
Check
BB
Click and drag to start drawing a structure.
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibility
Chapter 7 Solutions
Chemistry: Atoms First
Ch. 7 - What does it mean to say an equation is balanced?...Ch. 7 - Consider molecular, complete ionic, and net ionic...Ch. 7 - Balance the following equations: (a)...Ch. 7 - Balance the following equations: (a)...Ch. 7 - Write a balanced molecular equation describing...Ch. 7 - Write a balanced equation describing each of the...Ch. 7 - Colorful fireworks often involve the decomposition...Ch. 7 - Fill in the blank with a single chemical formula...Ch. 7 - Aqueous hydrogen fluoride (hydrofluoric acid) is...Ch. 7 - A novel process for obtaining magnesium from sea...
Ch. 7 - From the balanced molecular equations, write the...Ch. 7 - Use the following equations to answer the next...Ch. 7 - Indicate what type, or types, of reaction each of...Ch. 7 - Indicate what type, or types, of reaction each of...Ch. 7 - Silver can be separated from gold because silver...Ch. 7 - Determine the oxidation states of the elements in...Ch. 7 - Determine the oxidation states of the elements in...Ch. 7 - Determine the oxidation states of the elements in...Ch. 7 - Classify the following as acid-base reactions or...Ch. 7 - Identify the atoms that are oxidized and reduced,...Ch. 7 - Complete and balance the following acid-base...Ch. 7 - Complete and balance the following acid-base...Ch. 7 - Complete and balance the following...Ch. 7 - Complete and balance the following...Ch. 7 - Complete and balance the equations for the...Ch. 7 - When heated to 700—800 C, diamonds, which are pure...Ch. 7 - The military has experimented with lasers that...Ch. 7 - Write the molecular, total ionic, and net ionic...Ch. 7 - Great Lakes Chemical Company produces bromine,...Ch. 7 - In a common experiment in the general chemistry...Ch. 7 - Lithium hydroxide may be used to absorb carbon...Ch. 7 - Calcium propionate is sometimes added to bread to...Ch. 7 - Complete and balance the equations of the...Ch. 7 - Copper(II) sulfide is oxidized by molecular oxygen...Ch. 7 - Write balanced chemical equations for the...Ch. 7 - Calcium cyclamate Ca(C6H 11 NHSO3)2 is an...Ch. 7 - Complete and balance each of the following...Ch. 7 - Complete and balance each of the following...Ch. 7 - Balance each of the following equations according...Ch. 7 - Balance each of the following equations according...Ch. 7 - Balance each of the following equations according...Ch. 7 - Write the balanced equation, then outline the...Ch. 7 - Determine the number of moles and the mass...Ch. 7 - Write the balanced equation, then outline the...Ch. 7 - Determine the number of moles and the mass...Ch. 7 - H2 is produced by the reaction of 118.5 mL of a...Ch. 7 - Gallium chloride is formed by the reaction of 2.6...Ch. 7 - I2 is produced by the reaction of 0.4235 mol of...Ch. 7 - Silver is often extracted from ores such as...Ch. 7 - What mass of silver oxide, Ag2O, is required to...Ch. 7 - Carborundum is silicon carbide, SiC, a very hard...Ch. 7 - Automotive air bags inflate when a sample of...Ch. 7 - Urea, CO( NH2)2, is manufactured on a large scale...Ch. 7 - In an accident, a solution containing 2.5 kg of...Ch. 7 - A compact car gets 37.5 miles per gallon on the...Ch. 7 - What volume of 0.750 M hydrochloric acid solution...Ch. 7 - What volume of a 0.2089 M Kl solution contains...Ch. 7 - A mordant is a substance that combines with a dye...Ch. 7 - The toxic pigment called white lead, Pb3(OH)2(...Ch. 7 - The following quantities are placed in a...Ch. 7 - What is the limiting reactant in a reaction that...Ch. 7 - Which of the postulates of Dalton’s atomic theory...Ch. 7 - A student isolated 25 g of a compound following a...Ch. 7 - A sample of 0.53 g of carbon dioxide was obtained...Ch. 7 - Freon-12, CCl2F2, is prepared from CCl4 by...Ch. 7 - Citric acid, C6H5CH3, a component of jams,...Ch. 7 - Toluene, C6H5CH3, is oxidized by air under...Ch. 7 - In a laboratory experiment, the reaction of 3.0...Ch. 7 - Outline the steps needed to solve the following...Ch. 7 - Outline the steps needed to determine the limiting...Ch. 7 - Outline the steps needed to determine the limiting...Ch. 7 - What is the limiting reactant when 1.50 g of...Ch. 7 - Uranium can be isolated from its ores by...Ch. 7 - How many molecules of C2H4Cl2 can be prepared from...Ch. 7 - How many molecules of the sweetener saccharin can...Ch. 7 - The phosphorus pentoxide used to produce...Ch. 7 - Would you agree to buy 1 trillion...Ch. 7 - What volume of 0.0105-M HBr solution is required...Ch. 7 - Titration of a 20.0-mL sample of acid rain...Ch. 7 - What is the concentration of NaCl in a solution if...Ch. 7 - In a common medical laboratory determination of...Ch. 7 - Potatoes can be peeled commercially by soaking...Ch. 7 - A sample of gallium bromide, GaBr3, weighing 0....Ch. 7 - The principal component of mothballs is...Ch. 7 - A 0.025-g sample of a compound composed of boron...Ch. 7 - Sodium bicarbonate (baking soda), NaHCO3, can be...Ch. 7 - What volume of 0.600 M HCl is required to react...Ch. 7 - What volume of 0.08892 M HNO3 is required to react...Ch. 7 - What volume of a 0.3300-M solution of sodium...Ch. 7 - What volume of a 0.00945-M solution of potassium...Ch. 7 - A sample of solid calcium hydroxide, Ca(OH)2, is...Ch. 7 - What mass of Ca(OH)2 will react with 25.0 g of...Ch. 7 - How many milliliters of a 0.1500-M solution of KOH...Ch. 7 - Potassium acid phthalate, KNaC8H4O4, or KHP, is...Ch. 7 - The reaction of WCl6 with Al at ~400 C gives black...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Write a formula for each acid. a. hydrofluoric acid b. hydrocyanic acid c. chlorous acid
Introductory Chemistry (6th Edition)
Raw Oysters and Antacids: A Deadly Mix? The highly acidic environment of the stomach kills most bacteria before...
Microbiology with Diseases by Body System (5th Edition)
True or false? Some trails are considered vestigial because they existed long ago.
Biological Science (6th Edition)
Match the following cell types with their correct definition. _________Macrophage _________NK cell _________Eos...
Human Anatomy & Physiology (2nd Edition)
[14.110] The following mechanism has been proposed for the gas-phase reaction of chloroform (CHCI3) and chlorin...
Chemistry: The Central Science (14th Edition)
25. The 100 kg block in FIGURE EX7.25 takes 6.0 s to reach the floor after being released from rest. What is th...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name the structurearrow_forward> For each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that: 1. the rate of substitution doesn't depend on nucleophile concentration and 2. the products are a roughly 50/50 mixture of enantiomers. Substrate A Substrate B Faster Rate X CI (Choose one) (Choose one) CI Br Explanation Check Br (Choose one) C 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy A F10arrow_forwardHow to draw this mechanism for the foloowing reaction in the foto. thank youarrow_forward
- Predict the major products of the following organic reaction: Some important notes: CN A? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. No reaction. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Centerarrow_forwardDraw the major product of the following reaction. Do not draw inorganic byproducts. H3PO4 OHarrow_forwardPredict the major products of this organic reaction: HBr (1 equiv) Δ ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. Explanation Check X ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacyarrow_forward
- For the structure below, draw the resonance structure that is indicated by the curved arrow(s). Be sure to include formal charges. :ÖH Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds.arrow_forwardUsing the table of Reactants and Products provided in the Hints section, provide the major product (with the correct stereochemistry when applicable) for questions below by selecting the letter that corresponds to the exact chemical structures for the possible product. OH conc Hydrochloric acid 40°C Temp A/arrow_forwardUsing arrows to designate the flow of electrons, complete the reaction below and provide a detailed mechanism for the formation of the product OH conc Hydrochloric acid 40°C Temp All chemical structures should be hand drawn on a piece of paper Paragraph BI UAE +varrow_forward
- draw out the following structures plesearrow_forwardDraw everything on a piece of paper outlining the synthesis from acetaldehyde to 2 cyclopentene carboxaldehyde using carbon based reagants with 3 carbons or fewers. Here is the attached image.arrow_forwardManoharan Mariappan, FR.D., 34) Complete the following reaction starting from hex-1-yne proceeding via different substitution reactions forming 2-heptanone. (25 pts). A Sia₂BH H₂O₂ NaOH Br D Mechanism for reaction D - ether-cleavage: 10 B Ph-MgCI, THF H₁₂O+ D HBr (XS) C TsCl, Py CH3-CH2-CH2-ONaarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co


Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning
GCSE Chemistry - Differences Between Compounds, Molecules & Mixtures #3; Author: Cognito;https://www.youtube.com/watch?v=jBDr0mHyc5M;License: Standard YouTube License, CC-BY