
Interpretation:
3s, 3p and 3d orbitals which have the same energy in a hydrogen atom but different energies in a many-electron atom should be explained by using the concept of quantum numbers and Aufbau principle.
Concept Introduction:
Energies of orbitals in the hydrogen atom
The energies of orbitals in the hydrogen atom depend only on the value of the principal quantum number (n). When n increases, energy also increases. For this reason, orbitals in the same shell have the same energy in spite of their subshell. The increasing order of energy of hydrogen orbitals is
1s < 2s = 2p < 3s = 3p = 3d < 4s = 4p = 4d = 4f
In the case of one 2s and three 2p orbitals in the second shell, they have the same energy. In the third shell, all nine orbitals (one 3s, three 3p and five 3d) have the same energy. All sixteen orbitals (one 4s, three 4p, five 4d and seven 4f) in the fourth shell have the same energy.
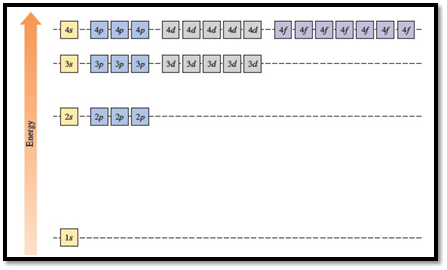
Figure 1
The energy levels of the different orbitals in hydrogen atom are easily explained by considering the given diagram. Here, each box represents one orbital. Orbitals with the same principal quantum number (n) have the same energy.
Energies of orbitals in a many electron atom
Energy of an orbital in a many electron atom depends on both the values of principle quantum number (n) and
Principal Quantum Number (n)
The principal quantum number (n) assigns the size of the orbital and specifies the energy of an electron. If the value of n is larger, then the average distance of an electron in the orbital from the nucleus will be greater. Therefore the size of the orbital is large. The principal quantum numbers have the integral values of 1, 2, 3 and so forth and it corresponds to the quantum number in
Angular Momentum Quantum Number (l)
The angular momentum quantum number (l) explains the shape of the atomic orbital. The values of l are integers which depend on the value of the principal quantum number, n. For a given value of n, the possible values of l range are from 0 to n − 1. If n = 1, there is only one possible value of l (l=0). If n = 2, there are two values of l: 0 and 1. If n = 3, there are three values of l: 0, 1, and 2. The value of l is selected by the letters s, p, d, and f. If l = 0, we have an s orbital; if l = 1, we have a p orbital; if l = 2, we have a d orbital and finally if l = 3, we have a f orbital. A collection of orbitals with the same value of n is called a shell. One or more orbitals with the same n and l values are referred to a subshell (sublevel). The value of l also has a slight effect on the energy of the subshell; the energy of the subshell increases with l (s < p < d < f).
Aufbau principle
In the ground state of an atom, an electron enters the orbital with lowest energy first and subsequent electrons are fed in the order of increasing energies. This is called Aufbau principle. The word 'aufbau' in German means 'building up'. Here, it refers to the filling up of orbitals with electrons. Aufbau principle explains the theoretical, sequential building up of the stepwise addition of protons and electrons for the elements in the periodic table.
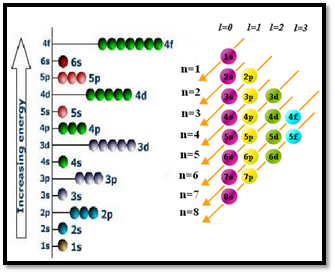
Figure 2
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
EBK GENERAL CHEMISTRY: THE ESSENTIAL CO
- १ eq ine teaching and × + rn/takeAssignment/takeCovalentActivity.do?locator-assignment-take [Review Topics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. The IUPAC name is In progress mit Answer Retry Entire Group 5 more group attempts remaining Cengage Learning | Cengage Technical Support Save and Exitarrow_forwardDraw the molecules.arrow_forwardDraw the mechanism for the acid-catalyzed dehydration of 2-methyl-hexan-2-ol with arrows please.arrow_forward
- . Draw the products for addition reactions (label as major or minor) of the reaction between 2-methyl-2-butene and with following reactants : Steps to follow : A. These are addition reactions you need to break a double bond and make two products if possible. B. As of Markovnikov rule the hydrogen should go to that double bond carbon which has more hydrogen to make stable products or major product. Here is the link for additional help : https://study.com/academy/answer/predict-the-major-and-minor-products-of-2-methyl- 2-butene-with-hbr-as-an-electrophilic-addition-reaction-include-the-intermediate- reactions.html H₂C CH3 H H3C CH3 2-methyl-2-butene CH3 Same structure CH3 IENCESarrow_forwardDraw everything on a piece of paper including every single step and each name provided using carbons less than 3 please.arrow_forwardTopics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. H The IUPAC name isarrow_forward
- [Review Topics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. The IUPAC name is Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forwardPlease draw.arrow_forwardA chromatogram with ideal Gaussian bands has tR = 9.0 minutes and w1/2 = 2.0 minutes. Find the number of theoretical plates that are present, and calculate the height of each theoretical plate if the column is 10 centimeters long.arrow_forward
- An open tubular column has an inner diameter of 207 micrometers, and the thickness of the stationary phase on the inner wall is 0.50 micrometers. Unretained solute passes through in 63 seconds and a particular solute emerges at 433 seconds. Find the distribution constant for this solute and find the fraction of time spent in the stationary phase.arrow_forwardConsider a chromatography column in which Vs= Vm/5. Find the retention factor if Kd= 3 and Kd= 30.arrow_forwardTo improve chromatographic separation, you must: Increase the number of theoretical plates on the column. Increase the height of theoretical plates on the column. Increase both the number and height of theoretical plates on the column. Increasing the flow rate of the mobile phase would Increase longitudinal diffusion Increase broadening due to mass transfer Increase broadening due to multiple paths You can improve the separation of components in gas chromatography by: Rasing the temperature of the injection port Rasing the temperature of the column isothermally Rasing the temperature of the column using temperature programming In GC, separation between two different solutes occurs because the solutes have different solubilities in the mobile phase the solutes volatilize at different rates in the injector the solutes spend different amounts of time in the stationary phasearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





