
Concept explainers
(a)
Interpretation:
The structure of all products formed in the given reaction is to be shown.
Concept Introduction:
The compounds which have same molecular formula but different connectivity of atoms are known as constitutional isomers. Chiral compounds are those compounds which contain an asymmetric carbon atom. Chiral molecules are optically active molecules. Stereocentre can be an atom, bond, or any point in molecule at which interchange of two groups form a stereoisomer.
Answer:
(1) The structures of all products formed in the given reactions are shown below.
(2) The structures of all products formed in the given reactions are shown below.
(3) The structures of all products formed in the given reactions are shown below.
(4) The structures of all products formed in the given reactions are shown below.
(5) The structures of all products formed in the given reactions are shown below.
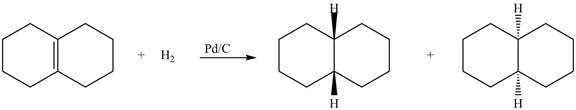
(6) The structures of all products formed in the given reactions are shown below.
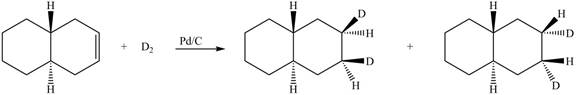
Explanation:
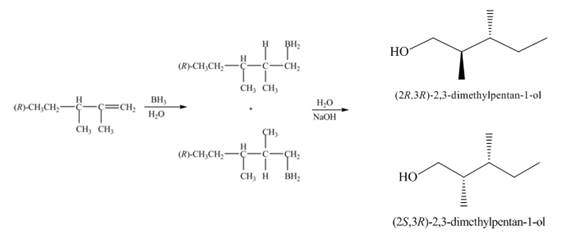
(1) The
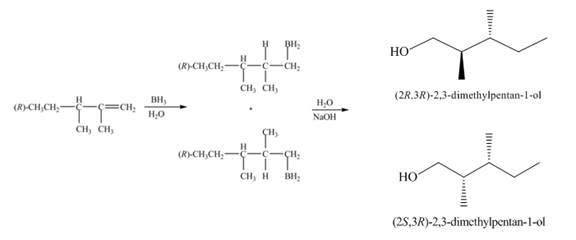
Figure 1
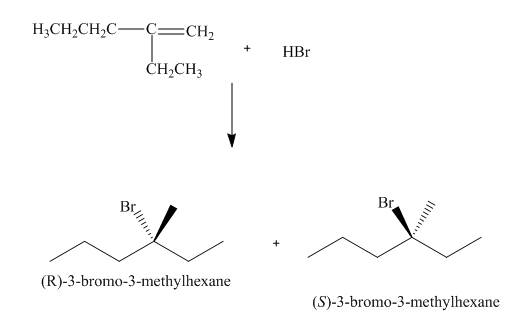
(2) The electrophilic addition of hydrogen bromide takes place on the carbon-carbon double bond of the alkene. The addition follows markonikov’s rule, the hydrogen atom goes to the less substituted carbon atom. The products formed in the given reaction are shown below.

Figure 2
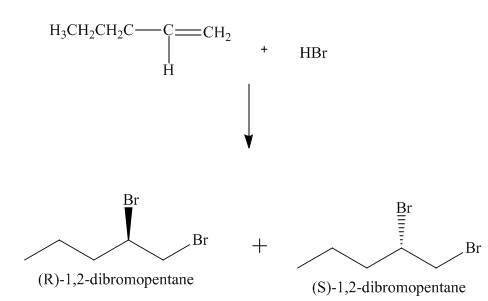
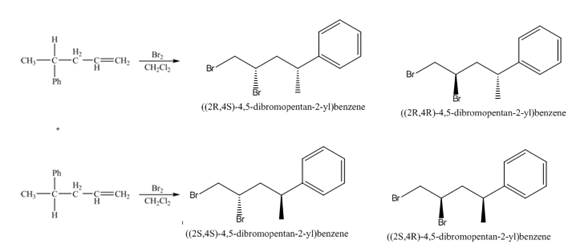
(3) The addition reaction of bromine on the alkene takes place. The addition of bromine on alkene is anti addition. The cyclic bromonium ion intermediate is formed in the reaction. The product formed in the given reaction is shown below.

Figure 3
(4) The addition reaction of bromine on the alkene takes place. The addition of bromine on alkene is anti addition. The cyclic bromonium ion intermediate is formed in the reaction. The product formed in the given reaction is shown below.
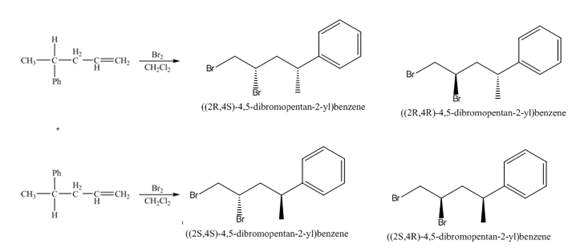
Figure 4
(5) In the reduction reaction of alkene in presence of palladium metal the hydrogen gets adsorbed on the metal surface. Alkene reduction to form
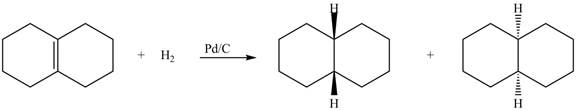
Figure 5
(6) In the reduction reaction of alkene in presence of palladium metal,
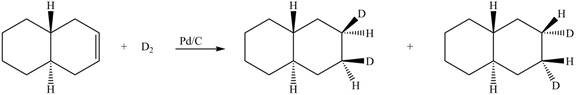
Figure 6
Conclusion:
The products formed in the given reactions are shown in Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6.
Answer to Problem 7.42AP
(1) The structures of all products formed in the given reactions are shown below.

(2) The structures of all products formed in the given reactions are shown below.

(3) The structures of all products formed in the given reactions are shown below.

(4) The structures of all products formed in the given reactions are shown below.

(5) The structures of all products formed in the given reactions are shown below.

(6) The structures of all products formed in the given reactions are shown below.

Explanation of Solution
(1) The alkene undergoes hydroboration-oxidation reaction to form alcohol compounds. The borane gets added to the double bond. Then it will undergo oxidation reaction to form the alcohol compound. The products formed in the given reaction are shown below.

Figure 1
(2) The electrophilic addition of hydrogen bromide takes place on the carbon-carbon double bond of the alkene. The addition follows markonikov’s rule, the hydrogen atom goes to the less substituted carbon atom. The products formed in the given reaction are shown below.

Figure 2
(3) The addition reaction of bromine on the alkene takes place. The addition of bromine on alkene is anti addition. The cyclic bromonium ion intermediate is formed in the reaction. The product formed in the given reaction is shown below.

Figure 3
(4) The addition reaction of bromine on the alkene takes place. The addition of bromine on alkene is anti addition. The cyclic bromonium ion intermediate is formed in the reaction. The product formed in the given reaction is shown below.

Figure 4
(5) In the reduction reaction of alkene in presence of palladium metal the hydrogen gets adsorbed on the metal surface. Alkene reduction to form alkane takes place forming syn addition product. The products formed in the given reaction are shown below.

Figure 5
(6) In the reduction reaction of alkene in presence of palladium metal,

Figure 6
The products formed in the given reactions are shown in Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6.
(b)
Interpretation:
The stereochemical relationship between products formed is to be stated.
Concept Introduction:
Stereoisomers which are non-superimposable and not mirror image are known as diastereomer. The compound must contain two or more than two stereocentre. Diastereomer are non identical stereoisomers. The pair of stereoisomer which are mirror image of each other are known as enantiomers. Enantiomers are non-congruent mirror images. If the molecules are placed on top of each other they will not give same molecule.
Answer to Problem 7.42AP
(1) The products formed are diastereomers.
(2) The products formed are enantiomers.
(3) The products formed are enantiomers.
(4) The two pairs of products formed are diastereomers.
(5) Only one product is formed.
(6) Only one product is formed.
Explanation of Solution
(1) The products formed are non-congruent and not mirror image of each other. Hence they are diastereomers.
(2) The products formed are non-congruent mirror image of each other. Therefore, they are enantiomers.
(3) The products formed are non-congruent mirror image of each other. Therefore, they are enantiomers.
(4) The products formed are non-congruent and not mirror image of each other. Hence they are diastereomers.
(5) Only one product is formed.
(6) Only one product is formed.
The stereochemical relationship between products formed in reaction (1) and (4) are diastereomers and reaction (2) and (3) are enantiomers.
(c)
Interpretation:
The products are formed in identical or different amount is to be stated.
Concept Introduction:
The electrophilic addition reaction on the alkene can takes place from any side of alkene. The electrophile can attack the carbon double bond from above or below the plane. The probability of attack from both sides is equal.
Answer to Problem 7.42AP
(1) The products are formed in equal amount.
(2) The products are formed in equal amount.
(3) The products are formed in equal amount.
(4) The products are formed in equal amount.
(5) Only one product is formed.
(6) Only one product is formed.
Explanation of Solution
(1) The alkene is a planar molecule. The reaction on the carbon-carbon double bond of alkene can takes place from above or below the plane. Therefore, products are formed in equal amount.
(2) The alkene is a planar molecule. The reaction on the carbon-carbon double bond of alkene can takes place from above or below the plane. Therefore, products are formed in equal amount.
(3) The alkene is a planar molecule. The reaction on the carbon-carbon double bond of alkene can takes place from above or below the plane. Therefore, products are formed in equal amount.
(4) The alkene is a planar molecule. The reaction on the carbon-carbon double bond of alkene can takes place from above or below the plane. Therefore, products are formed in equal amount.
(5) Only one product is formed.
(6) Only one product is formed.
The products are formed in equal amount in reaction (1), (2), (3), (4) and only one product is formed in reaction (5) and (6).
(d)
Interpretation:
The products which have different physical properties is to be stated.
Concept Introduction:
Stereoisomers which are non-superimposable and not mirror image are known as diastereomer. The compound must contain two or more than two stereocentre. Diastereomer are non identical stereoisomers. The pair of stereoisomer which are mirror image of each other are known as enantiomers. Enantiomers are non-congruent mirror images. Diastereomers shows different physical properties and enantiomers show same physical properties.
Answer to Problem 7.42AP
(1) The products formed in the given reaction will have different physical properties.
(2) The products formed in the given reaction will have identical physical properties.
(3) The products formed in the given reaction will have identical physical properties.
(4) The products formed in the given reaction will have different physical properties.
(5) Only one product is formed.
(6) Only one product is formed.
Explanation of Solution
(1) The products formed in the given reaction are diastereomer. Diastereomers show different physical properties. Therefore, the products formed in the given reaction will have different physical properties.
(2) The products formed in the given reaction are enantiomers. Enantiomers show identical physical properties. Therefore, the products formed in the given reaction will have identical physical properties.
(3) The products formed in the given reaction are enantiomers. Enantiomers show identical physical properties. Therefore, the products formed in the given reaction will have identical physical properties.
(4) The products formed in the given reaction are diastereomer. Diastereomers show different physical properties. Therefore, the products formed in the given reaction will have different physical properties.
(5) Only one product is formed.
(6) Only one product is formed.
The products formed in the reaction (1) and reaction (4) will have different physical properties. The products formed in the reaction (2) and reaction (3) will have identical physical properties. In reaction (5) and (6) only one product is formed.
Want to see more full solutions like this?
Chapter 7 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- Construct a molecular orbital energy-level diagram for BeH2. Sketch the MO pictures (schematic representation) for the HOMO and LUMO of BeH2 [Orbital Potential Energies, H (1s): -13.6 eV; Be (2s): -9.3 eV, Be (2p): -6.0 eV]arrow_forwardIndicate the isomers of the A(H2O)6Cl3 complex. State the type of isomerism they exhibit and explain it briefly.arrow_forwardState the formula of the compound potassium μ-dihydroxydicobaltate (III) tetraoxalate.arrow_forward
- Consider the reaction of the cyclopentanone derivative shown below. i) NaOCH2CH3 CH3CH2OH, 25°C ii) CH3!arrow_forwardWhat constitutes a 'reference material', and why does its utilization play a critical role in the chemical analysis of food products? Provide examples.arrow_forwardExplain what calibration is and why it is essential in relation to food analysis. Provide examples.arrow_forward
- The cobalt mu-hydroxide complex cobaltate(III) of potassium is a dinuclear complex. Correct?arrow_forwardThe cobalt mi-hydroxide complex cobaltate(III) of potassium is a dinuclear complex. Correct?arrow_forward3. Arrange the different acids in Exercise B # 2 from the strongest (1) to the weakest acid (10). 1. 2. (strongest) 3. 4. 5. 6. 7. 8. 9. 10 10. (weakest)arrow_forward
- Name Section Score Date EXERCISE B pH, pOH, pка, AND PKD CALCULATIONS 1. Complete the following table. Solution [H+] [OH-] PH РОН Nature of Solution A 2 x 10-8 M B 1 x 10-7 M C D 12.3 6.8 2. The following table contains the names, formulas, ka or pka for some common acids. Fill in the blanks in the table. (17 Points) Acid Name Formula Dissociation reaction Ka pka Phosphoric acid H₂PO₁ H3PO4 H++ H₂PO 7.08 x 10-3 Dihydrogen H₂PO H₂PO H+ HPO 6.31 x 10-6 phosphate Hydrogen HPO₁ 12.4 phosphate Carbonic acid H2CO3 Hydrogen HCO 6.35 10.3 carbonate or bicarbonate Acetic acid CH,COOH 4.76 Lactic acid CH₂CHOH- COOH 1.38 x 10 Ammonium NH 5.63 x 10-10 Phenol CH₂OH 1 x 10-10 Protonated form CH3NH3* 3.16 x 10-11 of methylaminearrow_forwardIndicate whether it is true that Co(III) complexes are very stable.arrow_forwardMnO2 acts as an oxidant in the chlorine synthesis reaction.arrow_forward
