
Concept explainers
A particle of mass m = 1.18 kg is attached between two identical springs on a frictionless, horizontal tabletop. Both springs have spring constant k and are initially unstressed, and the particle is at x = 0. (a) The particle is pulled a distance x along a direction perpendicular to the initial configuration of the springs as shown in Figure P7.50. Show that the force exerted by the springs on the particle is
(b) Show that the potential energy of the system is
(c) Make a plot of U(x) versus x and identify all equilibrium points. Assume L = 1.20 m and k = 40.0 N/m. (d) If the panicle is pulled 0.500 m to the right and then released, what is its speed when it reaches x = 0?
Figure P7.50
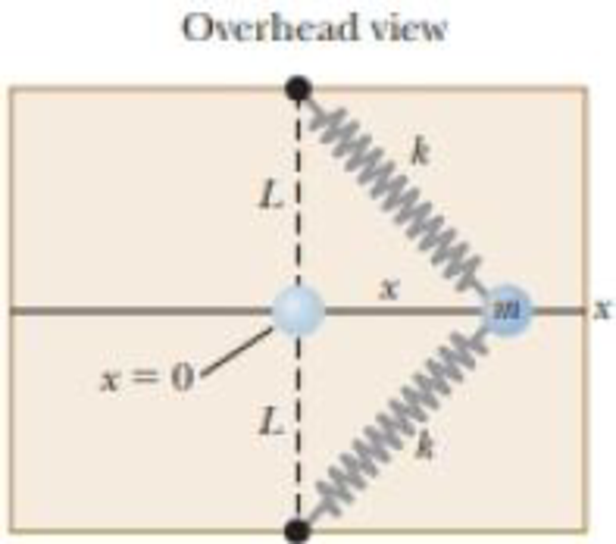
(a)
To show: The force exerted by the spring on the particle is
Answer to Problem 66CP
The force exerted by the spring on the particle is
Explanation of Solution
Given info: The mass of the particle is
The free body diagram of the given case is as shown in the figure below.
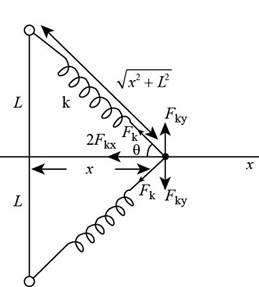
Figure (1)
The extension in the spring is,
Here,
The new length after stretching is,
Here,
Substitute
From the figure (1) the net force in
The net force in
The negative sign is due to the direction of the force in the negative direction.
Here,
From the free body diagram the value of
The formula for the spring force is,
Substitute
Substitute
Write the expression for the force exerted by the spring on the particle.
Substitute
Conclusion:
Therefore, the force exerted by the spring on the particle is
(b)
To show: The potential energy of the system is
Answer to Problem 66CP
The potential energy of the system is
Explanation of Solution
Given info: The mass of the particle is
From part (a) the force exerted by the spring on the particle is,
The potential energy of a system is,
Substitute
Conclusion:
Therefore, the potential energy of the system is
(c)
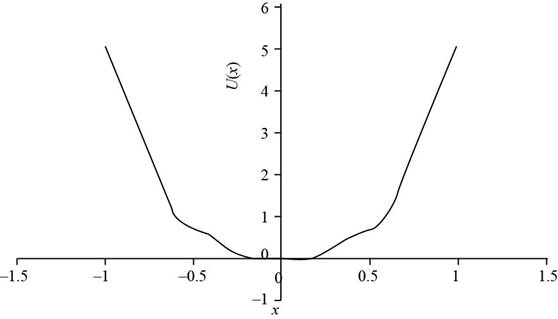
To draw: The plot of
Answer to Problem 66CP
The plot for
Explanation of Solution
Introduction:
The equilibrium points are the points at which the values of the force and the potential energy have the minimum value in order to have higher stability in the system.
Given info: The mass of the particle is
The potential energy of the system is,
Substitute
Thus, the potential energy of the system is,
The plot
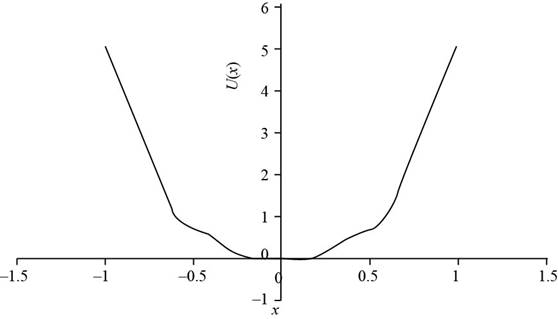
Figure (2)
The equilibrium points are the point at the potential energy is zero. In the above plot the minimum potential energy is at
Form the graph the equilibrium point is for
Conclusion:
Therefore, the plot for
(d)
The speed of the particle.
Answer to Problem 66CP
The speed of the particle is
Explanation of Solution
Given info: The mass of the particle is
The potential energy of the system is,
Substitute
Thus, the potential energy of the system is
The potential energy is converted in to the kinetic energy to follow the law of conservation of momentum.
Here,
Rearrange the above equation for
Substitute
Conclusion:
Therefore, speed of the particle is
Want to see more full solutions like this?
Chapter 7 Solutions
Physics for Scientists and Engineers With Modern Physics
Additional Science Textbook Solutions
College Physics: A Strategic Approach (3rd Edition)
Laboratory Manual For Human Anatomy & Physiology
SEELEY'S ANATOMY+PHYSIOLOGY
Fundamentals Of Thermodynamics
Chemistry: A Molecular Approach (4th Edition)
Chemistry & Chemical Reactivity
- Q: What is the direction of the magnetic field at point A, due to the current I in a wire, in each of the cases 1 to 6 shown below? Note: point A is in the plane of the page. ▪A I I ▪A (1) (2) ▪A • I (out of page) (3) ▪A I x I (into page) ▪A ▪A I (4) (5) (6)arrow_forwardA tennis ball is thrown into the air with initial speed vo=46 m/s and angle (theta) 38 degrees from the ground. Find the distance it travels (x) when it hits the ground.arrow_forwardProblem 04.08 (17 points). Answer the following questions related to the figure below. ථි R₁ www R₂ E R₁ www ли R₁ A Use Kirchhoff's laws to calculate the currents through each battery and resistor in terms of R1, R2, E1, & E2. B Given that all the resistances and EMFs have positive values, if E₁ > E2 and R₁ > R2, which direction is the current flowing through E₁? Through R₂? C If E1 E2 and R₁ > R2, which direction is the current flowing through E₁? Through R2?arrow_forward
- A 105- and a 45.0-Q resistor are connected in parallel. When this combination is connected across a battery, the current delivered by the battery is 0.268 A. When the 45.0-resistor is disconnected, the current from the battery drops to 0.0840 A. Determine (a) the emf and (b) the internal resistance of the battery. 10 R2 R₁ ww R₁ Emf 14 Emf Final circuit Initial circuitarrow_forwardA ball is shot at an angle of 60° with the ground. What should be the initial velocity of the ball so that it will go inside the ring 8 meters away and 3 meters high. Suppose that you want the ball to be scored exactly at the buzzer, determine the required time to throw and shoot the ball. Full solution and figure if there is.arrow_forwardCorrect answer please. I will upvote.arrow_forward
- Define operational amplifierarrow_forwardA bungee jumper plans to bungee jump from a bridge 64.0 m above the ground. He plans to use a uniform elastic cord, tied to a harness around his body, to stop his fall at a point 6.00 m above the water. Model his body as a particle and the cord as having negligible mass and obeying Hooke's law. In a preliminary test he finds that when hanging at rest from a 5.00 m length of the cord, his body weight stretches it by 1.55 m. He will drop from rest at the point where the top end of a longer section of the cord is attached to the bridge. (a) What length of cord should he use? Use subscripts 1 and 2 respectively to represent the 5.00 m test length and the actual jump length. Use Hooke's law F = KAL and the fact that the change in length AL for a given force is proportional the length L (AL = CL), to determine the force constant for the test case and for the jump case. Use conservation of mechanical energy to determine the length of the rope. m (b) What maximum acceleration will he…arrow_forward9 V 300 Ω www 100 Ω 200 Ω www 400 Ω 500 Ω www 600 Ω ww 700 Ω Figure 1: Circuit symbols for a variety of useful circuit elements Problem 04.07 (17 points). Answer the following questions related to the figure below. A What is the equivalent resistance of the network of resistors in the circuit below? B If the battery has an EMF of 9V and is considered as an ideal batter (internal resistance is zero), how much current flows through it in this circuit? C If the 9V EMF battery has an internal resistance of 2 2, would this current be larger or smaller? By how much? D In the ideal battery case, calculate the current through and the voltage across each resistor in the circuit.arrow_forward
- helparrow_forwardIf the block does reach point B, how far up the curved portion of the track does it reach, and if it does not, how far short of point B does the block come to a stop? (Enter your answer in m.)arrow_forwardTruck suspensions often have "helper springs" that engage at high loads. One such arrangement is a leaf spring with a helper coil spring mounted on the axle, as shown in the figure below. When the main leaf spring is compressed by distance yo, the helper spring engages and then helps to support any additional load. Suppose the leaf spring constant is 5.05 × 105 N/m, the helper spring constant is 3.50 × 105 N/m, and y = 0.500 m. Truck body yo Main leaf spring -"Helper" spring Axle (a) What is the compression of the leaf spring for a load of 6.00 × 105 N? Your response differs from the correct answer by more than 10%. Double check your calculations. m (b) How much work is done in compressing the springs? ☑ Your response differs significantly from the correct answer. Rework your solution from the beginning and check each step carefully. Jarrow_forward
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning





