
Concept explainers
Interpretation:
The stereochemical outcome of the given molecule treated with strong base to produce major product should be draw and identified.
Concept Introduction:
E2 elimination reaction: E2 elimination occurs in a compound when the leaving group is antiperiplaner to the β-proton in presence of strong base.
Stereospecific reaction: reaction undergoes from a stereoisomer to a unique stereoisomeric product.
Newman projection: Newman projection of molecule is one type of representations for the
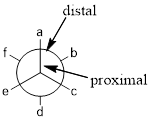
From the angle of observer the front carbon is proximal and second carbon is distal.
In wedge-dash line representation wedge is coming out of the plane and dash is going behind the plane, the wedge line and the dash line of front carbon in Newman projection are pointing up and the wedge line and the dash line of back carbon in Newman projection are pointing down.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
ORGANIC CHEMISTRY 1 TERM ACCESS
- What are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardhelp with the rf values i am so confusedarrow_forwardPredict the organic reactant of X and Y that are involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forward
- What are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardPlease provide the complete mechanism for the reaction below and include all appropriate arrows, formal charges, and intermediates. Please draw out the answerarrow_forwardPredict the major organic product for this reaction.arrow_forward
- help me with the rf value i am so confusedarrow_forwardPredict the major organic product for this reaction.arrow_forward3) The following molecule, chloral is a common precursor to chloral hydrate, an acetal type molecule that was a first-generation anesthetic. Draw a mechanism that accounts for tis formation and speculate why it does not require the use of an acid catalyst, like most hemiacetal and acetal reaction: (10 pts) H H₂Oarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





