
Identify each of the following unbalanced reaction equations as belonging to one or more of the following categories: precipitation, acid—base, or oxidation—reduction.
l type='a'>
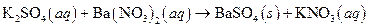
i>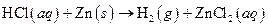
i>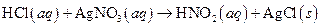
i>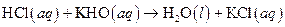
i>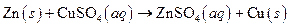
i>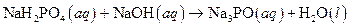
i>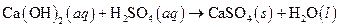
i>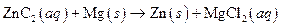
i>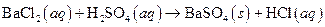
(a)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
Answer to Problem 53QAP
The given reaction is an example of precipitation reaction.
Explanation of Solution
Given:
The balance reaction is as follows:
This is an example of precipitation reaction because here a solid
(b)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
Answer to Problem 53QAP
The given reaction is an example of oxidation-reduction reaction.
Explanation of Solution
Given:
The balance reaction is as follows:
This is an example of oxidation −reduction reaction because in the above reaction oxidation number changed.
(c)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of precipitation reaction.
Given:
The balance reaction is as follows:
This is an example of precipitation reaction because here a solid
(d)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of acid -base reaction.
Given:
The balance reaction is as follows:
This is an example of acid −base or neutralization reaction because here salt and water will form.
(e)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of oxidation-reduction reaction.
Given:
The balance reaction is as follows:
This is an example of oxidation −reduction reaction because in the above reaction oxidation number changed.
(f)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of acid -base reaction.
Given:
The balance reaction is as follows:
This is an example of acid −base or neutralization reaction because here salt and water will form.
(g)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of acid -base reaction.
Given:
The balance reaction is as follows:
This is an example of acid −base or neutralization reaction because here salt and water will form.
(h)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of oxidation-reduction reaction.
Given:
The balance reaction is as follows:
This is an example of oxidation−reduction reaction because in the above reaction oxidation number changed.
i)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of precipitation reaction.
Given:
The balance reaction is as follows:
This is an example of precipitation reaction because here a solid
Answer to Problem 53QAP
The given reaction is an example of precipitation reaction.
Given:
The balance reaction is as follows:
This is an example of precipitation reaction because here a solid
(d)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of acid -base reaction.
Given:
The balance reaction is as follows:
This is an example of acid −base or neutralization reaction because here salt and water will form.
(e)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of oxidation-reduction reaction.
Given:
The balance reaction is as follows:
This is an example of oxidation −reduction reaction because in the above reaction oxidation number changed.
(f)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of acid -base reaction.
Given:
The balance reaction is as follows:
This is an example of acid −base or neutralization reaction because here salt and water will form.
(g)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of acid -base reaction.
Given:
The balance reaction is as follows:
This is an example of acid −base or neutralization reaction because here salt and water will form.
(h)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of oxidation-reduction reaction.
Given:
The balance reaction is as follows:
This is an example of oxidation−reduction reaction because in the above reaction oxidation number changed.
i)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of precipitation reaction.
Given:
The balance reaction is as follows:
This is an example of precipitation reaction because here a solid
Explanation of Solution
Given:
The balance reaction is as follows:
This is an example of precipitation reaction because here a solid
(d)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
Answer to Problem 53QAP
The given reaction is an example of acid -base reaction.
Explanation of Solution
Given:
The balance reaction is as follows:
This is an example of acid −base or neutralization reaction because here salt and water will form.
(e)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
Answer to Problem 53QAP
The given reaction is an example of oxidation-reduction reaction.
Explanation of Solution
Given:
The balance reaction is as follows:
This is an example of oxidation −reduction reaction because in the above reaction oxidation number changed.
(f)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
Answer to Problem 53QAP
The given reaction is an example of acid -base reaction.
Explanation of Solution
Given:
The balance reaction is as follows:
This is an example of acid −base or neutralization reaction because here salt and water will form.
(g)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
Answer to Problem 53QAP
The given reaction is an example of acid -base reaction.
Explanation of Solution
Given:
The balance reaction is as follows:
This is an example of acid −base or neutralization reaction because here salt and water will form.
(h)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
Answer to Problem 53QAP
The given reaction is an example of oxidation-reduction reaction.
Explanation of Solution
Given:
The balance reaction is as follows:
This is an example of oxidation−reduction reaction because in the above reaction oxidation number changed.
i)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
Answer to Problem 53QAP
The given reaction is an example of precipitation reaction.
Explanation of Solution
Given:
The balance reaction is as follows:
This is an example of precipitation reaction because here a solid
Want to see more full solutions like this?
Chapter 7 Solutions
Introductory Chemistry: A Foundation
- What is the molarisuty of a 0.396 m glucose solution if its density is 1.16 g/mL? MM glucose 180.2 /mol.arrow_forwardProvide the proper IUPAC or common name for the following compound. Dashes, commas, and spaces must be used correctly. Br ......Im OHarrow_forwardCan you please help me solve this problems. The top one is just drawing out the skeletal correct and then the bottom one is just very confusing to me and its quite small in the images. Can you enlarge it and explain it to me please. Thank You much (ME EX1) Prblm #33arrow_forward
- I'm trying to memorize VESPR Shapes to solve problems like those. I need help making circles like the second image in blue or using an x- and y-axis plane to memorize these and solve those types of problems, especially the ones given in the top/first image (180, 120, 109.5). Can you help me with this? or is their any other efficient method do soarrow_forwardCan you please explain this problems to me? I'm very confused about it. Please provide a detailed, step-by-step explanation for me! (ME EX1) Prblm 27arrow_forwardQuestion 6 of 7 (1 point) | Question Attempt: 1 of 1 = 1 ✓2 ✓ 3 ✓ 4 ✓ 5 6 ✓ 7 This organic molecule is dissolved in a basic aqueous solution: Jen ✓ ? A short time later sensitive infrared spectroscopy reveals the presence of a new C-OH stretch absorption. That is, must now be a new molecule present with at least one C- OH bond. there 18 In the drawing area below, show the detailed mechanism that could convert the molecule above into the new molecule Ar © + Click and drag to start drawing a structure. Add/Remove step Click and drawing Save For Later Submit Assignmentarrow_forward
- Can you please explain this problem to me? I'm very confused about it. Please provide a detailed, step-by-step explanation for me! (ME EX1) Prblm 22arrow_forwardCan you please explain this problems to me? I'm very confused about it. Please provide a detailed, step-by-step explanation for me! (ME EX1) Prblm 30arrow_forwardThis organic molecule is dissolved in a basic aqueous solution: O ? olo RET A short time later sensitive infrared spectroscopy reveals the presence of a new C-OH stretch absorption. That is, there Ar must now be a new molecule present with at least one C - OH bond. In the drawing area below, show the detailed mechanism that could convert the molecule above into the new molecule. $ Add/Remove steparrow_forward
- So the thing is im trying to memorize VESPR Shapes in order to be able to solve problems like so, and I need help with making circles like the second image that's in blue or using an x and y axis plane in order to memorize these and be able to solve those type of problems. Especially like the ones given in the top / first image. (180 , 120 , 109.5) Can you help me with this.arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward2. (15 points) Draw an appropriate mechanism for the following reaction. H N. H* + H₂Oarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning




