
Identify each of the following unbalanced reaction equations as belonging to one or more of the following categories: precipitation, acid—base, or oxidation—reduction.
l type='a'>
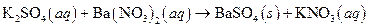
i>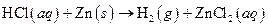
i>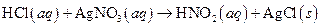
i>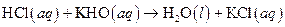
i>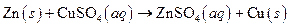
i>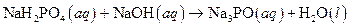
i>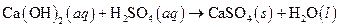
i>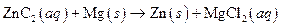
i>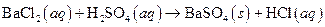
(a)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
Answer to Problem 53QAP
The given reaction is an example of precipitation reaction.
Explanation of Solution
Given:
The balance reaction is as follows:
This is an example of precipitation reaction because here a solid
(b)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
Answer to Problem 53QAP
The given reaction is an example of oxidation-reduction reaction.
Explanation of Solution
Given:
The balance reaction is as follows:
This is an example of oxidation −reduction reaction because in the above reaction oxidation number changed.
(c)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of precipitation reaction.
Given:
The balance reaction is as follows:
This is an example of precipitation reaction because here a solid
(d)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of acid -base reaction.
Given:
The balance reaction is as follows:
This is an example of acid −base or neutralization reaction because here salt and water will form.
(e)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of oxidation-reduction reaction.
Given:
The balance reaction is as follows:
This is an example of oxidation −reduction reaction because in the above reaction oxidation number changed.
(f)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of acid -base reaction.
Given:
The balance reaction is as follows:
This is an example of acid −base or neutralization reaction because here salt and water will form.
(g)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of acid -base reaction.
Given:
The balance reaction is as follows:
This is an example of acid −base or neutralization reaction because here salt and water will form.
(h)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of oxidation-reduction reaction.
Given:
The balance reaction is as follows:
This is an example of oxidation−reduction reaction because in the above reaction oxidation number changed.
i)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of precipitation reaction.
Given:
The balance reaction is as follows:
This is an example of precipitation reaction because here a solid
Answer to Problem 53QAP
The given reaction is an example of precipitation reaction.
Given:
The balance reaction is as follows:
This is an example of precipitation reaction because here a solid
(d)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of acid -base reaction.
Given:
The balance reaction is as follows:
This is an example of acid −base or neutralization reaction because here salt and water will form.
(e)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of oxidation-reduction reaction.
Given:
The balance reaction is as follows:
This is an example of oxidation −reduction reaction because in the above reaction oxidation number changed.
(f)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of acid -base reaction.
Given:
The balance reaction is as follows:
This is an example of acid −base or neutralization reaction because here salt and water will form.
(g)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of acid -base reaction.
Given:
The balance reaction is as follows:
This is an example of acid −base or neutralization reaction because here salt and water will form.
(h)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of oxidation-reduction reaction.
Given:
The balance reaction is as follows:
This is an example of oxidation−reduction reaction because in the above reaction oxidation number changed.
i)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
The given reaction is an example of precipitation reaction.
Given:
The balance reaction is as follows:
This is an example of precipitation reaction because here a solid
Explanation of Solution
Given:
The balance reaction is as follows:
This is an example of precipitation reaction because here a solid
(d)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
Answer to Problem 53QAP
The given reaction is an example of acid -base reaction.
Explanation of Solution
Given:
The balance reaction is as follows:
This is an example of acid −base or neutralization reaction because here salt and water will form.
(e)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
Answer to Problem 53QAP
The given reaction is an example of oxidation-reduction reaction.
Explanation of Solution
Given:
The balance reaction is as follows:
This is an example of oxidation −reduction reaction because in the above reaction oxidation number changed.
(f)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
Answer to Problem 53QAP
The given reaction is an example of acid -base reaction.
Explanation of Solution
Given:
The balance reaction is as follows:
This is an example of acid −base or neutralization reaction because here salt and water will form.
(g)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
Answer to Problem 53QAP
The given reaction is an example of acid -base reaction.
Explanation of Solution
Given:
The balance reaction is as follows:
This is an example of acid −base or neutralization reaction because here salt and water will form.
(h)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
Answer to Problem 53QAP
The given reaction is an example of oxidation-reduction reaction.
Explanation of Solution
Given:
The balance reaction is as follows:
This is an example of oxidation−reduction reaction because in the above reaction oxidation number changed.
i)
Interpretation:
To complete and balance the given combustion reaction.
Concept Introduction:
A balanced chemical equation is an equation that contains same number of atoms as well as of each element of reactants and products of reaction.
For example, the reaction between lead sulphide and oxygen is as follows:
Oxidation-reduction reaction is known as redox reaction. In these types of reaction one reactant is oxidized and another is reduced.
Oxidation: Oxidation is a process in which either 1 or all following changes occurs:
- Gaining of oxygen atoms.
- Loss of electrons.
- Loss of hydrogen atom.
Reduction: Reduction is a process in which either 1 or all following changes occurs:
- Loss of oxygen atoms.
- Gaining of electrons.
- Gaining of hydrogen atom.
Precipitation reaction means formation of solids or formation of any Precipitate; when solutions of two ionic substances are mixed and any solid will forms in the solution mixture, the reaction is known as Precipitation reaction. The reaction of barium hydroxide and sodium sulphate is an example of precipitate reaction, where barium sulphate a precipitate is formed as follows:
When an acid is reacted with base creating water or solvent and an ionic salt, the reaction is known as Acid-base neutralization reaction for example the reaction of strong acid, hydrochloric acid with strong base sodium hydroxide is as follows:
Answer to Problem 53QAP
The given reaction is an example of precipitation reaction.
Explanation of Solution
Given:
The balance reaction is as follows:
This is an example of precipitation reaction because here a solid
Want to see more full solutions like this?
Chapter 7 Solutions
Introductory Chemistry: A Foundation
- Name these organic compounds: structure Br name CH3 CH3 ☐ ☐arrow_forwardHH H-C H -C-H HH Draw the Skeletal Structures & H Name the molecules HH H H H H-C-C-C-C-C-C-H HHH HHH H H HHHHHHH H-C-C-C-C-C-C-C-C-C-H HHHHH H H H Harrow_forwarddont provide AI solution .... otherwise i will give you dislikearrow_forward
- Name these organic compounds: structure name CH3 CH3 ☐ F F CH3 ☐ O Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terms ofarrow_forwardClassify each of the following molecules as aromatic, antiaromatic, or nonaromatic. ZI NH Explanation Check O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic H O nonaromatic O aromatic O antiaromatic O nonaromatic ×arrow_forwardPart I. Draw the stepwise reaction mechanism of each product (a, b, c, d, e, f) HO HO OH НОН,С HO OH Sucrose HO CH₂OH H N N HO -H H -OH KMnO4, Heat H OH CH₂OH (d) Phenyl Osatriazole OH НОН,С HO HO + Glacial HOAC HO- HO CH₂OH OH HO Fructose (a) Glucose OH (b) H₂N HN (c) CuSO4-5H2O, ethanol H N N N HO ·H H OH H OH N CH₂OH OH (f) Phenyl Osazone H (e) Carboxy phenyl osatriazole Figure 2.1. Reaction Scheme for the Total Synthesis of Fine Chemicalsarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning




