
Concept explainers
Write structural formulas for each of the following:
Vinylcycloheptane
Interpretation:
The structural formula for each of the given compounds is to be written.
Concept introduction:
When writing the structural formula of any compound, first the functional group from the suffix of the given name is identified.
The longest carbon chain containing the functional group is located.
The carbon atoms of the chain are numbered in a such way that the functional group is at the lowest numbered carbon atom.
Substituents are attached to the parent chain according to their positions given in the name.
In alkenes, the Z isomers have the higher ranked substituents on the same side of the double bond, and in E isomers, higher ranked substituents are on opposite sides of the double bond.
Answer to Problem 31P
Solution:
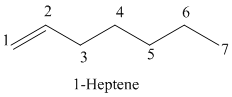
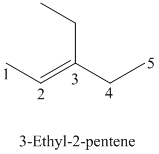
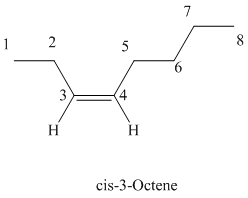
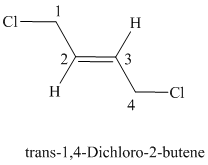
Explanation of Solution
a)
The parent chain has seven carbon atoms since the name has the word “hept.” The functional group is alkene. The double bond is present between
The structural formula is as follows:
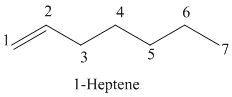
b)
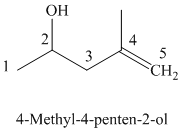
The parent chain has five carbon atoms since the name has the word “pent.” The functional group is alkene. The double bond is present between
The structural formula is as follows:
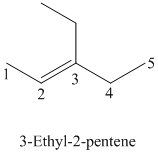
c)
The parent chain has eight carbon atoms since the name has the word “oct.” The functional group is alkene. The double bond is present between
The structural formula is as follows:
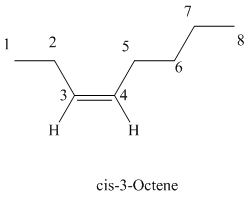
d)
The parent chain has four carbon atoms since the name has the word “but.” The functional group is alkene. The double bond is present between
The structural formula is as follows:
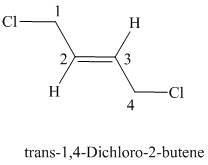
e)
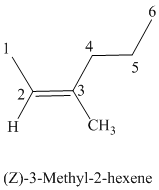
The parent chain has six carbon atoms since the name has the word “hex.” The functional group is alkene. The double bond is present between
The higher ranked substituents attached to the double bonded carbon atoms must be on the same side of the double bond since the conformation is Z. The structural formula is as follows:
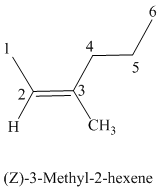
f)
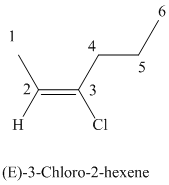
The parent chain has six carbon atoms since the name has the word “hex.” The functional group is alkene. The double bond is present between
The higher ranked substituents attached to the double bonded carbon atoms must be on the opposite side of the double bond since the conformation is E.
The structural formula is as follows:
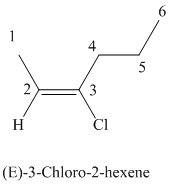
g)
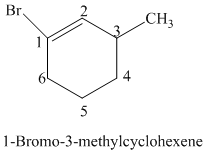
The parent is a six carbon atom ring since the name has the word “cyclohex.” The functional group is alkene. The double bond is present between
The structural formula is as follows:
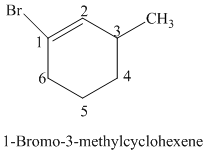
h)
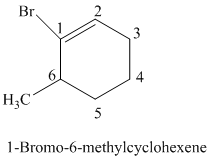
The parent is a six carbon atom ring since the name has the word “cyclohex.” The functional group is alkene. The double bond is present between
The structural formula is as follows:
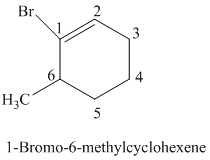
i)
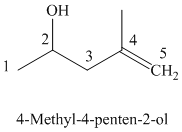
The parent chain has five carbon atoms since the name has the word “pent.” A double bond is present between
The structural formula is as follows:
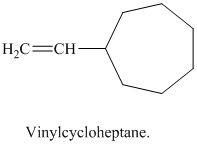
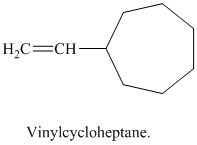
j) Vinylcycloheptane
The structure of a vinyl group is
A cycloheptane ring is attached to this vinyl group. The ring has seven carbon atoms. The structural formula is as follows:
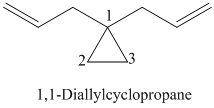
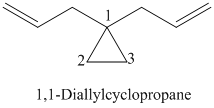
k)
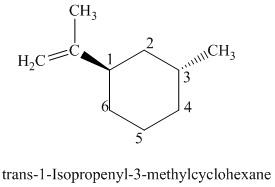
l)
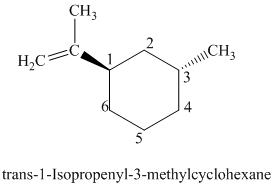
The parent is a six carbon atom ring since the name has the word “cyclohex.” An isopropenyl group is attached to the
The structural formula is as follows:
Want to see more full solutions like this?
Chapter 7 Solutions
ORGANIC CHEMISTRY (LOOSELEAF)-PACKAGE
- Draw the mechanism of the reaction.arrow_forward9. Draw all of the possible Monochlorination Products that would Result From the Free Radical Chlormation OF 23,4-TRIMethyl Pentane b. Calculate the To Yield For the major • Product given the Following Relative Restritus For 1° 2° and 30 Hydrogens toward Free Radical Chloration 5.0: 38 : 1 30 2° 1° C. what would be the major product in the Free Radical brominator Of the Same Molecule. Explain your Reasoning.arrow_forwardWhat is the complete reaction mechanism for the chlorination of Ethane, C2H6?arrow_forward
- A 13C NMR spectrum is shown for a molecule with the molecular formula of C6H100. Draw the structure that best fits this data. 220 200 180 160 140 120100 80 60 40 20 Drawingarrow_forwardPlease help me figure out the blan areas with step by step calculations.arrow_forwardneeding help draw all of the possible monochlorination products that would result from the free radical chlorination of 2,3,4-trimethylpentanearrow_forward
- HAND DRAWarrow_forwardBased on the 1H NMR, 13C NMR, DEPT 135 NMR and DEPT 90 NMR, provide a reasoning step and arrive at the final structure of an unknown organic compound containing 7 carbons. Dept 135 shows peak to be positive at 128.62 and 13.63 Dept 135 shows peak to be negative at 130.28, 64.32, 30.62 and 19.10. Provide assignment for the provided structurearrow_forwardO Predict the 'H NMR integration ratio for the following structure. IV I. 3 H A II. 1 H III. 2 H IV. 3 H I. 3 H B II. O H III. 2 H IV. 3 H I. 3 H C II. 2 H III. 2 Harrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning



