
INTRO.TO GENERAL,ORGAN...-OWLV2 ACCESS
12th Edition
ISBN: 9781337915977
Author: Bettelheim
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 7, Problem 31P
7-41 The equilibrium constant at 1127°C for the following endothermic reaction is 571:
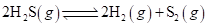
If the mixture is at equilibrium, what happens to K if we:
(a) Add some H2S?
(b) Add some H2?
(c) Lower the temperature to 1000°C?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
PLEASE HELP ! URGENT!
Identify priority of the substituents:
CH3
How many chiral carbons are in the molecule?
OH
F
CI
Br
Chapter 7 Solutions
INTRO.TO GENERAL,ORGAN...-OWLV2 ACCESS
Ch. 7.1 - Problem 7-1 In the reaction we measure the...Ch. 7.4 - Problem 7-2 Calculate the rate for the reaction in...Ch. 7.6 - Prob. 7.3QCCh. 7.6 - Prob. 7.4QCCh. 7.6 - Prob. 7.5QCCh. 7.7 - Prob. 7.6QCCh. 7.7 - Problem 7-7 Consider the following equilibrium...Ch. 7.7 - Prob. 7.8QCCh. 7.7 - Prob. 7.9QCCh. 7 - 7-11 Consider the following reaction: Suppose we...
Ch. 7 - 7-12 Two kinds of gas molecules are reacted at a...Ch. 7 - 7-13 Why are reactions between ions in aqueous...Ch. 7 - Prob. 4PCh. 7 - 7-15 A certain reaction is exothermic by 9...Ch. 7 - 7-16 A quart of milk quickly spoils if left at...Ch. 7 - 7-17 If a certain reaction takes 16 h to go to...Ch. 7 - Prob. 8PCh. 7 - Prob. 9PCh. 7 - Prob. 10PCh. 7 - Prob. 11PCh. 7 - 7-22 If you add a piece of marble, CaCO3 to a 6 M...Ch. 7 - Prob. 13PCh. 7 - Prob. 14PCh. 7 - Prob. 15PCh. 7 - 7-26 Write the chemical equations corresponding to...Ch. 7 - Prob. 17PCh. 7 - 7-28 When the following reaction reached...Ch. 7 - 7-29 The following reaction was allowed to reach...Ch. 7 - Prob. 20PCh. 7 - 7-31 Here are equilibrium constants for several...Ch. 7 - 7-32 A particular reaction has an equilibrium...Ch. 7 - Prob. 23PCh. 7 - Prob. 24PCh. 7 - 7-35 A reaction has a high rate constant but a...Ch. 7 - 7-36 Complete the following table showing the...Ch. 7 - Prob. 27PCh. 7 - Prob. 28PCh. 7 - Prob. 29PCh. 7 - 7-40 Is there any change in conditions that change...Ch. 7 - 7-41 The equilibrium constant at 1127°C for the...Ch. 7 - Prob. 32PCh. 7 - 7-43 (Chemical Connections 7A and 7B) Why is a...Ch. 7 - Prob. 34PCh. 7 - 7-45 (Chemical Connections 7C) A painkiller—for...Ch. 7 - 7-46 (Chemical Connections 7D) What reaction takes...Ch. 7 - Prob. 37PCh. 7 - Prob. 38PCh. 7 - Prob. 39PCh. 7 - 7-50 Draw an energy diagram for an exothermic...Ch. 7 - Prob. 41PCh. 7 - Prob. 42PCh. 7 - Prob. 43PCh. 7 - Prob. 44PCh. 7 - Prob. 45PCh. 7 - Prob. 46PCh. 7 - 7-57 Write the reaction to which the following...Ch. 7 - Prob. 48PCh. 7 - Prob. 49PCh. 7 - Prob. 50PCh. 7 - Prob. 51PCh. 7 - Prob. 52PCh. 7 - Prob. 53PCh. 7 - Prob. 54PCh. 7 - Prob. 55PCh. 7 - Prob. 56PCh. 7 - Prob. 57PCh. 7 - Prob. 58PCh. 7 - 7-69 Pure carbon exists is several forms, two of...Ch. 7 - Prob. 60PCh. 7 - 7-71 You have a beaker that contains solid silver...Ch. 7 - Prob. 62PCh. 7 - Prob. 63PCh. 7 - Prob. 64PCh. 7 - Prob. 65PCh. 7 - Prob. 66PCh. 7 - Prob. 67PCh. 7 - Prob. 68PCh. 7 - Prob. 69PCh. 7 - Prob. 70PCh. 7 - Prob. 71PCh. 7 - Prob. 72PCh. 7 - Prob. 73PCh. 7 - Prob. 74PCh. 7 - 7-82 An equilibrium mixture of O2, SO2, and SO3...Ch. 7 - Prob. 76PCh. 7 - Prob. 77P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forwardHow many chiral carbons are in the molecule? Farrow_forwardcan someone give the curly arrow mechanism for this reaction written with every intermediate and all the side products pleasearrow_forward
- I need the nomenclature of this compound.arrow_forwardI need the nomenclature of this compoundarrow_forward2. Name the following hydrocarbons. (9 marks) a) HHHHHHHH H-C-C- H-O-S b) HCEC-CH3 H H H H H d) c) H C=C- H H H e) CH3 CH3 CH2CH=CH-CH=CHCH3 HHHH H-C-C-C-C-H H HH H f) large CH2CH3 pola H3C section lovels tower, able ocart firs g) Tower H3C-CH2 then in H3C-CH-CH-CH3 enblbano bne noitsidab Copyright © 2008. Durham Continuing Education CH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Chemical Equilibria and Reaction Quotients; Author: Professor Dave Explains;https://www.youtube.com/watch?v=1GiZzCzmO5Q;License: Standard YouTube License, CC-BY