
Concept explainers
What is the major product obtained from the reaction of each of the following compounds with excess HCl?
- a. CH3CH2C≡CH
- b. CH3CH2C≡CCH2CH3
- c. CH3CH2C≡CCH2CH2CH3
(a)
Interpretation:
The major product obtained from the reaction of given compound with excess of
Concept Introduction:
Addition of halides to alkynes: Halogens like chlorine and bromine adds to the alkyne compounds. The general form of the reaction is given below.
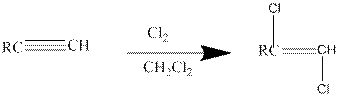
If excess halides are present, a second addition reaction also occurs.
Answer to Problem 29P
The major product obtained from the reaction of given compound with excess of

Explanation of Solution
Hydrogen halides undergo addition reaction with alkynes. Alkyne is a nucleophile and it reacts with the electrophile to give a

In this reaction of butyne with
(b)
Interpretation:
The major product obtained from the reaction of given compound with excess of
Concept Introduction:
Addition of halides to alkynes: Halogens like chlorine and bromine adds to the alkyne compounds. The general form of the reaction is given below.
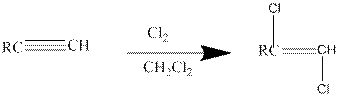
If excess halides are present, a second addition reaction also occurs.
Answer to Problem 29P
The major product obtained from the reaction of given compound with excess of

Explanation of Solution
Hydrogen halides undergo addition reaction with alkynes. Alkyne is a nucleophile and it reacts with the electrophile to give a

In this reaction of hex-3-yne with
(c)
Interpretation:
The major product obtained from the reaction of given compound with excess of
Concept Introduction:
Addition of halides to alkynes: Halogens like chlorine and bromine adds to the alkyne compounds. The general form of the reaction is given below.
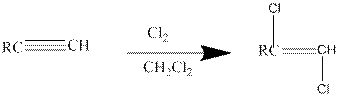
If excess halides are present, a second addition reaction also occurs.
Answer to Problem 29P
The major product obtained from the reaction of given compound with excess of
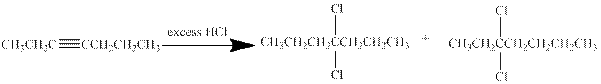
Explanation of Solution
Hydrogen halides undergo addition reaction with alkynes. Alkyne is a nucleophile and it reacts with the electrophile to give a
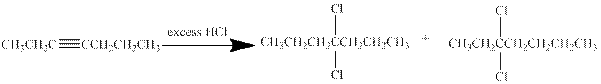
In this reaction of hept-3-yne with
Want to see more full solutions like this?
Chapter 7 Solutions
Organic Chemistry (8th Edition)
- For the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forwardName the following molecules with IUpacarrow_forward
- What is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forwardHow to get the predicted product of this reaction belowarrow_forward
- Please help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forwardProvide solutionsarrow_forward
- Which of these compounds is Ester formed from the reaction of acetic acid and one propanol arrow_forwardDescribe the four labeled parts of the reaction diagram from the reaction of sucrose, breaking down with and without an enzyme.arrow_forwardHow to determine the product with mechanism showedarrow_forward
