
Concept explainers
(a1)
To explain: The basis of the procedure for determining the number of
Introduction:
Carbohydrates are present in different forms. The carbohydrate made up of only one kind of sugar is monosaccharide, if formed of two types of sugars it is called disaccharide and if made up of many types of sugar it is polysaccharide.
(a1)
Explanation of Solution
The carbon number 6 of glucose residue present in
Pictorial representation: Fig 1: represents the structure of 2,3-di-O-methylglucose.
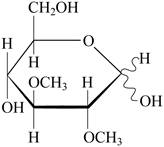
Fig 1: Structure of 2,3-di-O-methylglucose.
(a2)
To explain: What happens to the unbranched glucose residues in amylopectin during the methylation and hydrolysis procedure.
Introduction:
Carbohydrates are present in different forms. The carbohydrate made up of only one kind of sugar is monosaccharide, if formed of two types of sugars it is called disaccharide and if made up of many types of sugar it is polysaccharide.
(a2)
Explanation of Solution
The polysaccharides are large
In case of unbranched residue, methylation and hydrolysis process would yield 2,3,6-tri-O-methylglucose, as the hydroxyl group at carbon 2, 3, and 6 are free.
(b)
To calculate: The percentage of glucose residues in amylopectin contained
Introduction:
The polysaccharides are large polymer molecules composed of monosaccharides unit. Starch is an example of polysaccharide that are made up of amylose and amylopectin molecule. Amylose is a linear chain of starch that are compose of glucose units. Each glucose unit in amylose are joined through α-glycosidic bond. Whereas, amylopectin is branched structure of starch that contain β-glycosidic bond.
Given: 258 mg or
(b)
Explanation of Solution
The average molecular weight of glucose is 162g/mol, then
Thus, the total weight of amylopectin in
The weight of branched residue of 2,3-di-O-methylglucose in
Thus, the average molecular weight of glucose in 2,3-di-O-methylglucose is
The percentage of glucose residue glucose residue present in amylopectin having
The weight of branched residue is
Now, substitute the value in the above given equation and calculate the percentage of glucose residue in amylopectin.
Thus, the percentage of glucose residue in amylopectin having
The percentage of glucose residue in amylopectin is 3.75 %.
Want to see more full solutions like this?
Chapter 7 Solutions
Lehninger Principles of Biochemistry
- write the ionization equilibrium for cysteine and calculate the piarrow_forwardplease answerarrow_forwardf. The genetic code is given below, along with a short strand of template DNA. Write the protein segment that would form from this DNA. 5'-A-T-G-G-C-T-A-G-G-T-A-A-C-C-T-G-C-A-T-T-A-G-3' Table 4.5 The genetic code First Position Second Position (5' end) U C A G Third Position (3' end) Phe Ser Tyr Cys U Phe Ser Tyr Cys Leu Ser Stop Stop Leu Ser Stop Trp UCAG Leu Pro His Arg His Arg C Leu Pro Gln Arg Pro Leu Gin Arg Pro Leu Ser Asn Thr lle Ser Asn Thr lle Arg A Thr Lys UCAG UCAC G lle Arg Thr Lys Met Gly Asp Ala Val Gly Asp Ala Val Gly G Glu Ala UCAC Val Gly Glu Ala Val Note: This table identifies the amino acid encoded by each triplet. For example, the codon 5'-AUG-3' on mRNA specifies methionine, whereas CAU specifies histidine. UAA, UAG, and UGA are termination signals. AUG is part of the initiation signal, in addition to coding for internal methionine residues. Table 4.5 Biochemistry, Seventh Edition 2012 W. H. Freeman and Company B eviation: does it play abbreviation:arrow_forward
- Answer all of the questions please draw structures for major productarrow_forwardfor glycolysis and the citric acid cycle below, show where ATP, NADH and FADH are used or formed. Show on the diagram the points where at least three other metabolic pathways intersect with these two.arrow_forwardanswer the questions please all of them should be answeredarrow_forward
- Burk plot is shown below. Calculate Km and max for this enzyme. show workarrow_forwardInsert Format Tools Extensions Help Normal text ▾ Arial C 2 10 3 + BIUA Student Guide (continued) Record data and conclusions about the mystery food sample either below or in a lab notebook. Step 2: Protein Test (Biuret Solution) Gelatin Water [Mystery Food (Positive Control) (Negative Control) Sample pink purple no change no change They mystery food sample does not contain protein because the color of the test tube wasn't pink or purple Color Conclusion They mystery food sample does not contain protein because the color of the test tube wasn't pink or purple Step 3: Lipid Test (Sudan Red Solution) Vegetable Oil Water (Positive Control) (Negative Control) Mystery Food Sample floating red no change floating red the mystery food dosnt contain lipids because the test tube has floating red 75 % 87 8 9 7 ChromeOS C Device will pow 26.battery lea powerarrow_forwardThe rate data from an enzyme catalyzed reaction with and without an inhibitor present is found in the image. Question: what is the KM and Vm and the nature of inhibitionarrow_forward
- 1. Estimate the concentration of an enzyme within a living cell. Assume that: (a): fresh tissue is 80% water and all of it is intracellular (b): the total soluble protein represents 15% of the weight (c): all the soluble proteins are enzymes (d): the average molecular weight of the proteins is 150,000 (E): about 100 different enzymes are present please help I am lostarrow_forwardPlease helparrow_forwardThe following data were recorded for the enzyme catalyzed conversion of S -> P. Question: Estimate the Vmax and Km. What would be the rate at 2.5 and 5.0 x 10-5 M [S] ?arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





