
Concept explainers
Interpretation:
The IUPAC names of each of the given compounds are to be given by using
Concept introduction:
The priority of groups in nomenclature is assigned according to Cahn-Ingold-Prelog (CIP Rule) convention rules:
The higher the
If priority cannot be assigned according to atomic mass, then assign the priority according to first point of difference.
If both the priority groups are on the same side of double-bonded carbon atom, then it is known as
But, if both the priority groups are diagonal to each other, then it is
Rule for R/S Nomenclature:
4B(+)3B(-)2B(+)1B(-)
4B(+) means if 4th priority group is below then from 1 to 2 to 3 if leads a clockwise rotation then its R and if anticlockwise then it is S.
3B(-) means if 3rd priority group is below then from 1 to 2 to 4 if leads a clockwise rotation then its S and if anticlockwise then it is R.
Answer to Problem 1PP
Solution:
Explanation of Solution
a)
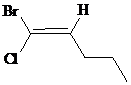
The IUPAC name with
Assign the priority to four groups, –Br gets first priority, pentyl group gets second, –Cl gets third, and –H gets fourth. Since both the priority groups are diagonal to each other, it is
Select the longest carbon chain. The parent hydrocarbon chain is pentane and hence pent- will be used and double bond is at the second position. Therefore, pent-1-ene is used.
Chloro and bromo groups are at first position. So, 1-bromo-1-chloropent-1-ene will be used.
Hence, the IUPAC name of the compound will be
b)
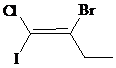
The IUPAC name with
Assign the priority to four groups, –I gets first priority, –Br gets second, –Cl gets third, and ethyl group gets fourth. Since both the priority groups are diagonal to each other, it is
Select the longest carbon chain. The parent hydrocarbon chain is butyl and hence but- will be used and double bond is at the first position. Therefore, but-1-ene is used.
Chloro and iodo groups are at first position and bromo group is at second position. So, 2-bromo-1-chloro-1-iodobut-1-ene will be used.
Hence, the IUPAC name of the compound will be
c)
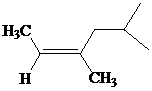
The IUPAC name with
Assign the priority to four groups, 2-methylpropyl gets first priority, both methyl groups get second, and –H gets third. Since both the priority groups are on the same side, it is
Select the longest carbon chain. The parent hydrocarbon chain is hexyl and hence hex- will be used and double bond is at the second position. Therefore, hex-2-ene is used.
There are two methyl groups at third and fifth positions. So, 3,5-dimethylhex-2-ene will be used.
Hence, the IUPAC name of the compound will be
d)
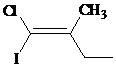
The IUPAC name with
Assign the priority to four groups, –I gets first priority, –Cl gets second, ethyl group gets third, and methyl group gets fourth. Since both the priority groups are on the same side, it is
Select the longest carbon chain. The parent hydrocarbon chain is butyl and hence but- will be used and double bond is at the first position. Therefore, but-1-ene is used.
There is one methyl group at second position, and chloro and iodo groups at first position. So, 1-chloro-1-iodo-2 methylbut-1-ene will be used.
Hence, the IUPAC name of the compound will be
e)
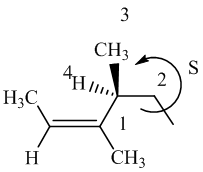
The IUPAC name with
Assign the priority to four groups, 1-methylpropyl gets first priority, both methyl groups get second, and –H gets third. Since both the priority groups are on the same side, it is
Select the longest carbon chain. The parent hydrocarbon chain is hexyl and hence hex- will be used and double bond is at the second position. Therefore, hex-2-ene is used.
There are two methyl groups at third and fourth position. So, 3,4-dimethylhex-2-ene will be used.
Carbon-4 is a chiral carbon. Assigning priority to the groups, 2-butene gets first priority, ethyl gets second, methyl gets third, and hydrogen gets fourth.
Move 1-2-3, it is in anticlockwise direction. So, it is
Hence, the IUPAC name of the compound will be
f)
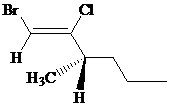
The IUPAC name can be done using the following steps:
Assign the priority to four groups, –Br gets first priority, –Cl gets second, 1-methylbutyl gets third, and –H gets fourth. Since both the priority groups are on the same side, it is
Select the longest carbon chain. The parent hydrocarbon chain is hexyl and hence hex- will be used and double bond is at the first position. Therefore, hex-1-ene is used.
There is one methyl group at third position, and chloro at second and bromo at first position. So, 1-bromo-2-chloro-3-methylhex-1-ene will be used.
Carbon-3 is a chiral carbon. Assigning priority to the groups, 1-bromo-2-chloroethene gets first priority, propyl gets second, methyl gets third, and hydrogen gets fourth.
Rotate the molecule such that –H is at a horizontal position and then move 1-2-3, it is in anticlockwise direction. So, it is
Hence, the IUPAC name of the compound will be
Want to see more full solutions like this?
Chapter 7 Solutions
ORGANIC CHEMISTRY-WILEYPLUS ACCESS PKG.
- esc 2 Incorrect Feedback: Your answer is incorrect. Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? A O • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. . If your answer is no, check the box under the drawing area instead. Check F1 ! @ X C Save For Later Submit Assignment 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 80 et A ད 1 4 F2 F3 F4 F5 F6 F7 F8 F9 F10 F11 F12 # $ 45 % A 6 87 & * 8 9 ) 0 + ||arrow_forwardCan the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ?A Δ O • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilit ku F11arrow_forward१ eq ine teaching and × + rn/takeAssignment/takeCovalentActivity.do?locator-assignment-take [Review Topics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. The IUPAC name is In progress mit Answer Retry Entire Group 5 more group attempts remaining Cengage Learning | Cengage Technical Support Save and Exitarrow_forward
- Draw the molecules.arrow_forwardDraw the mechanism for the acid-catalyzed dehydration of 2-methyl-hexan-2-ol with arrows please.arrow_forward. Draw the products for addition reactions (label as major or minor) of the reaction between 2-methyl-2-butene and with following reactants : Steps to follow : A. These are addition reactions you need to break a double bond and make two products if possible. B. As of Markovnikov rule the hydrogen should go to that double bond carbon which has more hydrogen to make stable products or major product. Here is the link for additional help : https://study.com/academy/answer/predict-the-major-and-minor-products-of-2-methyl- 2-butene-with-hbr-as-an-electrophilic-addition-reaction-include-the-intermediate- reactions.html H₂C CH3 H H3C CH3 2-methyl-2-butene CH3 Same structure CH3 IENCESarrow_forward
- Draw everything on a piece of paper including every single step and each name provided using carbons less than 3 please.arrow_forwardTopics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. H The IUPAC name isarrow_forward[Review Topics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. The IUPAC name is Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forward
- Please draw.arrow_forwardA chromatogram with ideal Gaussian bands has tR = 9.0 minutes and w1/2 = 2.0 minutes. Find the number of theoretical plates that are present, and calculate the height of each theoretical plate if the column is 10 centimeters long.arrow_forwardAn open tubular column has an inner diameter of 207 micrometers, and the thickness of the stationary phase on the inner wall is 0.50 micrometers. Unretained solute passes through in 63 seconds and a particular solute emerges at 433 seconds. Find the distribution constant for this solute and find the fraction of time spent in the stationary phase.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





