
(a)
Interpretation: The sequence of arrow pushing patterns of the curved arrows is to be interpreted from the given multistep reactions:
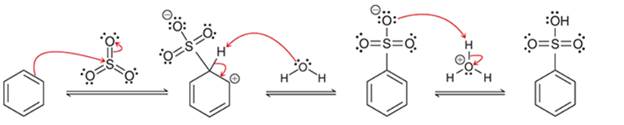
Concept introduction:
A
(b)
Interpretation: The sequence of arrow pushing patterns of the curved arrows is to be interpreted for the given multistep reactions.
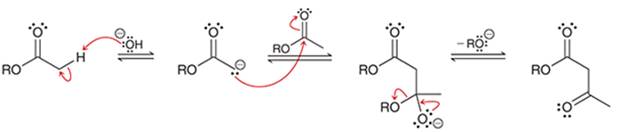
Concept introduction:
A chemical reaction can be completed in more than one step. Such reactions are called multistep reactions. It occurs with the formation of intermediates. The formation of intermediate can be shown with the help of curved arrows. Intermediates like carbocation and arenium cations are common intermediates.
(c)
Interpretation: The sequence of arrow pushing patterns of the curved arrows is to be interpreted for the given multistep reactions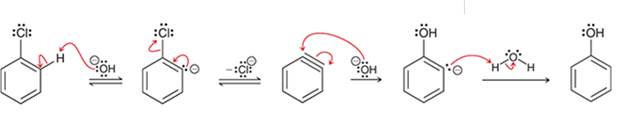
Concept introduction:
A chemical reaction can be completed in more than one step. Such reactions are called multistep reactions. It occurs with the formation of intermediates. The formation of intermediate can be shown with the help of curved arrows. Intermediates like carbocation and arenium cations are common intermediates.
(d)
Interpretation: The sequence of arrow pushing patterns of the curved arrows is to be interpreted for the given multistep reactions.
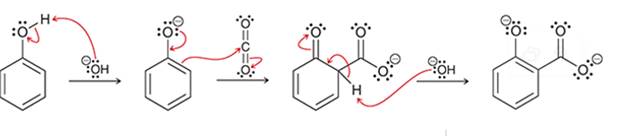
Concept introduction:
A chemical reaction can be completed in more than one step. Such reactions are called multistep reactions. It occurs with the formation of intermediates. The formation of intermediate can be shown with the help of curved arrows. Intermediates like carbocation and arenium cations are common intermediates.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
ORGANIC CHEM PRINT STUDY GDE & SSM
- Would the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward(a) Sketch the 'H NMR of the following chemical including the approximate chemical shifts, the multiplicity (splitting) of all signals and the integration (b) How many signals would you expect in the 13C NMR? CH3arrow_forwardDraw the Show the major and minor product(s) for the following reaction mechanisms for both reactions and show all resonance structures for any Explain why the major product is favoured? intermediates H-Brarrow_forward
- 3. Draw ALL THE POSSBILE PRODUCTS AND THE MECHANISMS WITH ALL RESONANCE STRUCTURES. Explain using the resonance structures why the major product(s) are formed over the minor product(s). H₂SO4, HONO CHarrow_forward7. Provide the product(s), starting material(s) and/or condition(s) required for the No mechanisms required. below reaction HO + H-I CI FO Br2, FeBr3 O I-Oarrow_forward6. Design the most efficient synthesis of the following product starting from phenot Provide the reaction conditions for each step (more than one step is required) and explain the selectivity of each reaction. NO MECHANISMS ARE REQUIRED. OH step(s) CIarrow_forward
- What is the skeletal structure of the product of the following organic reaction?arrow_forwardIf a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.arrow_forwardWhat is the major organic product of the following nucleophilic acyl substitution reaction of an acid chloride below?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

