
(a)
Interpretation: The sequence of arrow pushing patterns of the curved arrows is to be interpreted from the given multistep reactions:
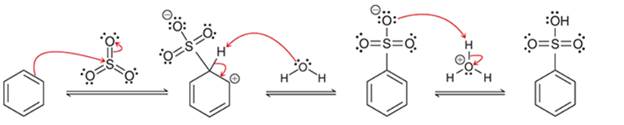
Concept introduction:
A
(b)
Interpretation: The sequence of arrow pushing patterns of the curved arrows is to be interpreted for the given multistep reactions.
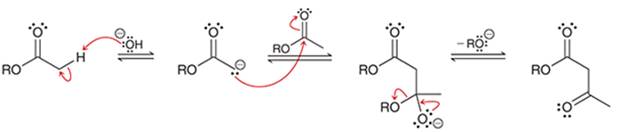
Concept introduction:
A chemical reaction can be completed in more than one step. Such reactions are called multistep reactions. It occurs with the formation of intermediates. The formation of intermediate can be shown with the help of curved arrows. Intermediates like carbocation and arenium cations are common intermediates.
(c)
Interpretation: The sequence of arrow pushing patterns of the curved arrows is to be interpreted for the given multistep reactions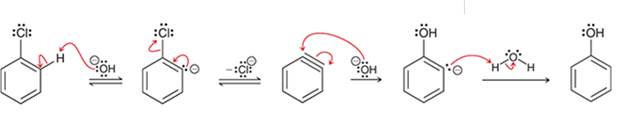
Concept introduction:
A chemical reaction can be completed in more than one step. Such reactions are called multistep reactions. It occurs with the formation of intermediates. The formation of intermediate can be shown with the help of curved arrows. Intermediates like carbocation and arenium cations are common intermediates.
(d)
Interpretation: The sequence of arrow pushing patterns of the curved arrows is to be interpreted for the given multistep reactions.
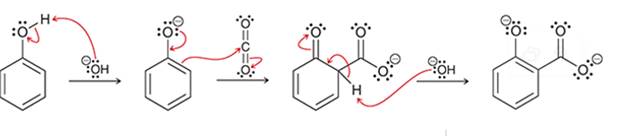
Concept introduction:
A chemical reaction can be completed in more than one step. Such reactions are called multistep reactions. It occurs with the formation of intermediates. The formation of intermediate can be shown with the help of curved arrows. Intermediates like carbocation and arenium cations are common intermediates.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
ORGANIC CHEMISTRY LL PRINT UPGRADE
- Which of the following metals is the only one with all of its bands completely full? Group of answer choices K Na Ca Alarrow_forward2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); Reagents: H₂O (B); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); HBr (1); R₂BH (6); H2SO4 (2); CH3OH (C); Br₂ (3); H₂O₂ / HO- (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI OH - α-α Br + enant Solvent Reagent(s) Solvent Reagent(s)arrow_forwardBased on concepts from Lecture 3-5, which of the following ionic compounds should be most soluble in water? Group of answer choices MgO BeO CaO BaOarrow_forward
- From an energy standpoint, which two process - in the correct order - are involved in the dissolving of an ionic compound crystal? Group of answer choices Water coordination to the ions followed by sublimation into the gas phase Sublimation of the crystal into gas-phase ions followed by water coordination to the ions Ion dissociation from the crystal followed by water coordination to the ions Water coordination to the ions followed by ion dissociation from the crystalarrow_forwardFor which Group 2 metal (M), is this process the most exothermic? M2+(g) + O2−(g) + CO2(g) → MO(s) + CO2(g) Group of answer choices M = Sr M = Mg M = Ca M = Baarrow_forward2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); H₂O (B); Reagents: HBr (1); H2SO4 (2); CH3OH (C); Br₂ (3); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); R₂BH (6); H₂O₂ / HO- (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI Solvent Reagent(s) Solvent Reagent(s) HO OHarrow_forward
- For which of the following ionic compounds would you expect the smallest difference between its theoretical and experimental lattice enthalpies? (You may assume these all have the same unit cell structure.) Electronegativities: Ca (1.0), Fe (1.8), Mg (1.2), O (3.5), S (2.5), Zn (1.6) Group of answer choices ZnO MgS CaO FeSarrow_forwardIn the Born-Haber cycle for KCl crystal formation, what enthalpy component must be divided by two? Group of answer choices KCl(s) enthalpy of formation Ionization energy for K(g) K(s) sublimation enthalpy Cl2 bond dissociation enthalpyarrow_forward2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); H₂O (B); Reagents: HBr (1); R₂BH (6); H2SO4 (2); CH3OH (C); Br₂ (3); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); H₂O₂ / HO (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI хот Br Solvent Reagent(s) Solvent Reagent(s)arrow_forward
- What is the correct chemical equation for the lattice formation reaction for CaBr2? Group of answer choices Ca2+(g) + 2 Br−(g) → CaBr2(s) ½ Ca2+(g) + Br−(g) → ½ CaBr2(s) Ca(s) + Br2(l) → CaBr2(s) Ca(s) + 2 Br−(g) → CaBr2(s)arrow_forwardPLEASE ANSWER THE QUESTION!!!arrow_forward3. SYNTHESIS. Propose a sequence of synthetic steps (FGI) that convert the starting material (SM) into the Target molecule. For each FGI in your proposed synthesis, specify the reagents / conditions, and draw the product(s) of that FGI. DO NOT INCLUDE the FGI mxn in the answer you submit. If an FGI requires two reagent sets, specify the order in which the reagent sets are added, e.g., i) Hg(OAc)2 / H₂O; ii) NaBH4/MeOH. Indicate the stereochemistry (if any) of the products of each FGI. FGI 1. Me Starting Material Source of all carbons in the Target molecule (can use multiple copies) Me Me Target molecule + enantiomerarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

