
ORGANIC CHEMISTRY-PRINT (LL)-W/WILEY
4th Edition
ISBN: 9781119761105
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 6.11, Problem 18ATS
Interpretation Introduction
Interpretation: In 1994, George Olah was awarded Nobel Prize for the research on carbocation. The rearrangement of carbocation he studied occurs with the migration of the methylene group. The curved arrow is to be interpreted for the migration of the methylene group in the given transformation.
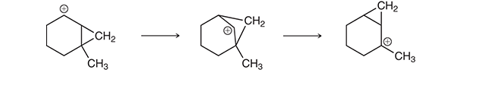
Concept introduction: In a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(ii)
Draw a reasonable mechanism for the following reaction:
CI
NaOH
heat
OH
(hint: SNAr Reaction)
:
Draw the major product in each of the following reaction:
Draw the mechanism for the following Friedel-Craft reaction.
AlBr3
Br
Chapter 6 Solutions
ORGANIC CHEMISTRY-PRINT (LL)-W/WILEY
Ch. 6.1 - Prob. 1LTSCh. 6.1 - Prob. 1PTSCh. 6.1 - Prob. 2ATSCh. 6.2 - Prob. 3CCCh. 6.3 - Prob. 4CCCh. 6.3 - Prob. 5CCCh. 6.4 - Prob. 6CCCh. 6.6 - Prob. 7CCCh. 6.7 - Prob. 2LTSCh. 6.7 - Prob. 8PTS
Ch. 6.7 - Prob. 9PTSCh. 6.7 - Prob. 10ATSCh. 6.8 - Prob. 3LTSCh. 6.8 - Prob. 11PTSCh. 6.8 - Prob. 12ATSCh. 6.9 - Prob. 4LTSCh. 6.9 - Prob. 13PTSCh. 6.9 - Prob. 14ATSCh. 6.10 - Prob. 5LTSCh. 6.10 - Prob. 15PTSCh. 6.10 - Prob. 16ATSCh. 6.11 - Prob. 6LTSCh. 6.11 - Prob. 17PTSCh. 6.11 - Prob. 18ATSCh. 6 - Prob. 19PPCh. 6 - Prob. 20PPCh. 6 - Prob. 21PPCh. 6 - Prob. 22PPCh. 6 - Prob. 24PPCh. 6 - Prob. 25PPCh. 6 - Prob. 26PPCh. 6 - Prob. 27PPCh. 6 - Prob. 28PPCh. 6 - Prob. 29PPCh. 6 - Prob. 30PPCh. 6 - Prob. 31PPCh. 6 - Prob. 32PPCh. 6 - Prob. 33PPCh. 6 - Prob. 34PPCh. 6 - Prob. 35PPCh. 6 - Prob. 36PPCh. 6 - Prob. 37PPCh. 6 - Prob. 38PPCh. 6 - Prob. 39PPCh. 6 - Prob. 40PPCh. 6 - Prob. 41PPCh. 6 - Prob. 43ASPCh. 6 - Prob. 44ASPCh. 6 - Prob. 45ASPCh. 6 - Prob. 46ASPCh. 6 - Prob. 47ASPCh. 6 - Prob. 48ASPCh. 6 - Prob. 49ASPCh. 6 - Prob. 50IPCh. 6 - Prob. 51IPCh. 6 - Prob. 52IPCh. 6 - Prob. 53IPCh. 6 - Prob. 54IPCh. 6 - Prob. 55IPCh. 6 - Prob. 56IPCh. 6 - Prob. 57IPCh. 6 - Prob. 58IPCh. 6 - Prob. 59IPCh. 6 - Prob. 60IPCh. 6 - Prob. 61IPCh. 6 - Prob. 62CPCh. 6 - Prob. 64CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (a) Draw the structures of A and B in the following reaction. (i) NaNH2, NH3(1) A + B (ii) H3O+arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forwardConsider the following decomposition reaction of N2O5(g): For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 → NO2 + NO3 (K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Indicate whether the following rate expression is acceptable: d[N2O5] = -k₁[N₂O₂] + K¸₁[NO₂][NO3] - K¸[NO₂]³ dtarrow_forward
- In a reaction of A + B to give C, another compound other than A, B or C may appear in the kinetic equation.arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forwardGiven the reaction R + Q → P, indicate the rate law with respect to R, with respect to P and with respect to P.arrow_forward
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardk₁ Given the reaction A B, indicate k-1 d[A] (A). the rate law with respect to A: (B). the rate law with respect to B: d[B] dt dtarrow_forwardk₁ Given the reaction R₂ R + R, indicate k-1 (A). the rate law with respect to R2: (B). the rate law with respect to R: d[R₂] dt d[R] dtarrow_forward
- Given the reaction R+ Q → P, indicate (A). the rate law with respect to P: (B). the rate law with respect to R: (C). the rate law with respect to Q: d[P] dt d[R] dt d[Q] dtarrow_forwardThe reaction for obtaining NO2 from NO and O2 has the rate equation: v = k[NO]2[O2]. Indicate which of the following options is correct.(A). This rate equation is inconsistent with the reaction consisting of a single trimolecular step.(B). Since the overall order is 3, the reaction must necessarily have some trimolecular step in its mechanism.(C). A two-step mechanism: 1) NO + NO ⇄ N2O2 (fast); 2) N2O2 + O2 → NO2 + NO2 (slow).(D). The mechanism must necessarily consist of three unimolecular elementary steps with very similar rate constants.arrow_forwarda. What is the eluent used in the column chromatography here (a “silica plug filtration” is essentially a very short column)? b. The spectroscopy of compound 5b is described in the second half of this excerpt, including 1H-NMR and 13C-NMR (which you will learn about in CHEM 2412L), MS (which you will learn about later in CHEM 2411L) and IR. One of the IR signals is at 3530 cm-1. What functional group does this indicate might be present in compound 5b?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY