
(a)
Interpretation:
The major products of the following reactions should be determined.
Concept Introduction:
Halogen addition to an alkene:
The addition reaction of halides to
Mainly
In general, the reaction can be shown as below.
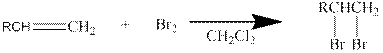
When the bromine approaches the alkene, one bromine accepts the electrons from alkene and give them to the other bromine and a cyclic bromonium ion intermediate is formed.
The formed unstable intermediate reacts with
(b)
Interpretation:
The major products of the following reactions should be determined.
Concept Introduction:
Halogen addition to an alkene:
The addition reaction of halides to alkenes leads to the formation of vicinal dihalide as the product. The two halogens will be on the adjacent carbons.
Mainly
In general, the reaction can be shown as below.
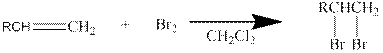
When the bromine approaches the alkene, one bromine accepts the electrons from alkene and give them to the other bromine and a cyclic bromonium ion intermediate is formed.
The formed unstable intermediate reacts with
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
ORGANIC CHEMISTRY-W/S.G+SOLN.MANUAL
- Basic strength of organic bases.arrow_forwardNucleophilic Aromatic Substitution: What is the product of the reaction? What is the name of the intermediate complex? *See imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor” *see attachedarrow_forward
- Nucleophilic Aromatic Substitution: What is the product of the reaction? *see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardThe answer here says that F and K have a singlet and a doublet. The singlet and doublet are referring to the H's 1 carbon away from the carbon attached to the OH. Why don't the H's two carbons away, the ones on the cyclohexane ring, cause more peaks on the signal?arrow_forward
- Draw the Birch Reduction for this aromatic compound and include electron withdrawing groups and electron donating groups. *See attachedarrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see imagearrow_forward
- Elimination-Addition: What molecule was determined to be an intermediate based on a “trapping experiment”? *please solve and see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor”. **see attachedarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
