
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
12th Edition
ISBN: 9780321908445
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 6, Problem 6.82UTC
Interpretation Introduction
To determine:
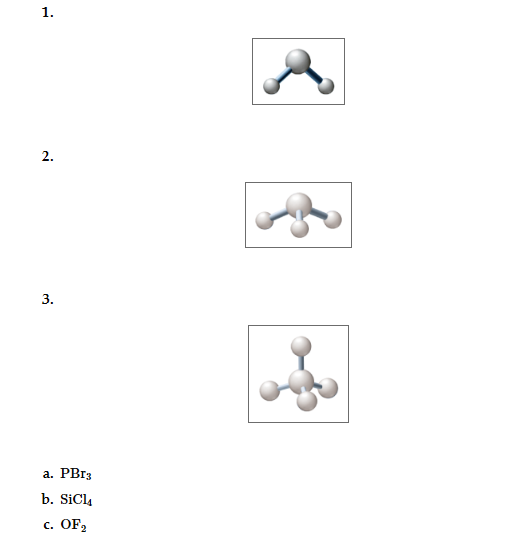
The given Lewis structure (a to c) with correct diagram (1 to 3) of its shapes and names of the shapes also indicate each molecule is polar or non-polar, assuming X and Y are non-metals.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the a-anomer cyclized pyranose Haworth projection of the below hexose. Circle the
anomeric carbons. Number the carbons on the Fischer and Haworth projections. Assign R
and S for each chiral center.
HO
CHO
-H
HO
-H
H-
-OH
H
-OH
CH₂OH
Draw the ẞ-anomer cyclized furanose Haworth projection for the below hexose. Circle the
anomeric carbons. Number the carbons on the Fischer and Haworth projections.
HO
CHO
-H
H
-OH
HO
-H
H
-OH
CH₂OH
Name the below disaccharide. Circle any hemiacetals. Identify the numbering of glycosidic
linkage, and identify it as a or ẞ.
OH
HO
HO
OH
HO
HO
HO
OH
What are the monomers used to make the following polymers?
F.
а.
b.
с.
d.
Вецер
хочому
な
Chapter 6 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Ch. 6.1 - State the number of electrons that be must be lost...Ch. 6.1 - State the number of electrons that must be gained...Ch. 6.1 - State the number of electrons lost or gained when...Ch. 6.1 - Prob. 6.4QAPCh. 6.1 - Write the symbols for the ions with the following...Ch. 6.1 - Write the symbols for the ions with the following...Ch. 6.1 - Write the symbol for the ion of each of the...Ch. 6.1 - Write the symbol for the ion of each of the...Ch. 6.2 - Which of the following pairs of elements are...Ch. 6.2 - Which of the following pairs of elements are...
Ch. 6.2 - Write the correct ionic formula for the compound...Ch. 6.2 - Write the correct ionic formula for the compound...Ch. 6.2 - Write the symbols for the ions, and the correct...Ch. 6.2 - Write the symbols for the ions, and the correct...Ch. 6.3 - Prob. 6.15QAPCh. 6.3 - Prob. 6.16QAPCh. 6.3 - Write the name for each of the following ions...Ch. 6.3 - Prob. 6.18QAPCh. 6.3 - Write the name for each of the following ionic...Ch. 6.3 - Prob. 6.20QAPCh. 6.3 - Prob. 6.21QAPCh. 6.3 - Prob. 6.22QAPCh. 6.3 - Prob. 6.23QAPCh. 6.3 - Write the formula for each of the following ionic...Ch. 6.3 - Prob. 6.25QAPCh. 6.3 - Prob. 6.26QAPCh. 6.4 - Write the formula including the charge for each of...Ch. 6.4 - Prob. 6.28QAPCh. 6.4 - Prob. 6.29QAPCh. 6.4 - Prob. 6.30QAPCh. 6.4 - Prob. 6.31QAPCh. 6.4 - Prob. 6.32QAPCh. 6.4 - Write the correct formula for the following ionic...Ch. 6.4 - Write the correct formula for the following ionic...Ch. 6.4 - Prob. 6.35QAPCh. 6.4 - Prob. 6.36QAPCh. 6.5 - Prob. 6.37QAPCh. 6.5 - Prob. 6.38QAPCh. 6.5 - Prob. 6.39QAPCh. 6.5 - Prob. 6.40QAPCh. 6.5 - Name each of the following molecular compounds:...Ch. 6.5 - Prob. 6.42QAPCh. 6.5 - Prob. 6.43QAPCh. 6.5 - Name each of the following molecular compounds: a....Ch. 6.5 - Write the formula for each of the following...Ch. 6.5 - Write the formula for each of the following...Ch. 6.5 - Write the formula for each of the following...Ch. 6.5 - Write the formula for each of the following...Ch. 6.5 - Prob. 6.49QAPCh. 6.5 - Prob. 6.50QAPCh. 6.6 - Describe the trend in electronegativity as...Ch. 6.6 - Prob. 6.52QAPCh. 6.6 - Using the periodic table, arrange the atoms in...Ch. 6.6 - Using the periodic table, arrange the atoms in...Ch. 6.6 - Predict whether each of the following bonds is...Ch. 6.6 - Predict whether each of the following bonds is...Ch. 6.6 - For each of the following bonds, indicate the...Ch. 6.6 - For each of the following bonds, indicate the...Ch. 6.7 - Choose the shape (1 to 6) that matches each of the...Ch. 6.7 - Prob. 6.60QAPCh. 6.7 - Prob. 6.61QAPCh. 6.7 - Complete each of the following statements for a...Ch. 6.7 - Prob. 6.63QAPCh. 6.7 - Prob. 6.64QAPCh. 6.7 - Prob. 6.65QAPCh. 6.7 - Prob. 6.66QAPCh. 6.7 - Prob. 6.67QAPCh. 6.7 - Prob. 6.68QAPCh. 6.7 - Prob. 6.69QAPCh. 6.7 - Prob. 6.70QAPCh. 6.8 - Prob. 6.71QAPCh. 6.8 - Prob. 6.72QAPCh. 6.8 - Prob. 6.73QAPCh. 6.8 - Prob. 6.74QAPCh. 6 - Prob. 6.75UTCCh. 6 - a. How does the octet rule explain the formation...Ch. 6 - Prob. 6.77UTCCh. 6 - Prob. 6.78UTCCh. 6 - Prob. 6.79UTCCh. 6 - Prob. 6.80UTCCh. 6 - Prob. 6.81UTCCh. 6 - Prob. 6.82UTCCh. 6 - Prob. 6.83UTCCh. 6 - Prob. 6.84UTCCh. 6 - Prob. 6.85UTCCh. 6 - 6.102 State the number of valence electrons,...Ch. 6 - Prob. 6.87AQAPCh. 6 - Prob. 6.88AQAPCh. 6 - Prob. 6.89AQAPCh. 6 - Prob. 6.90AQAPCh. 6 - Prob. 6.91AQAPCh. 6 - Prob. 6.92AQAPCh. 6 - Prob. 6.93AQAPCh. 6 - Prob. 6.94AQAPCh. 6 - Prob. 6.95AQAPCh. 6 - Prob. 6.96AQAPCh. 6 - Prob. 6.97AQAPCh. 6 - 6.120 Write the formula for each of the following...Ch. 6 - Prob. 6.99AQAPCh. 6 - Prob. 6.100AQAPCh. 6 - Prob. 6.101AQAPCh. 6 - Prob. 6.102AQAPCh. 6 - Prob. 6.103AQAPCh. 6 - Prob. 6.104AQAPCh. 6 - Prob. 6.105AQAPCh. 6 - Prob. 6.106AQAPCh. 6 - Prob. 6.107AQAPCh. 6 - Prob. 6.108AQAPCh. 6 - Prob. 6.109AQAPCh. 6 - Prob. 6.110AQAPCh. 6 - Prob. 6.111AQAPCh. 6 - Prob. 6.112AQAPCh. 6 - Prob. 6.113AQAPCh. 6 - Prob. 6.114AQAPCh. 6 - Prob. 6.115CQCh. 6 - Prob. 6.116CQCh. 6 - Prob. 6.117CQCh. 6 - Prob. 6.118CQCh. 6 - Prob. 6.119CQCh. 6 - Prob. 6.120CQCh. 6 - Prob. 7CICh. 6 - Prob. 8CICh. 6 - Prob. 9CICh. 6 - Prob. 10CICh. 6 - Prob. 11CICh. 6 - Of much concern to environmentalists is radon-222,...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. Propose a reasonable mechanism for the following transformation. I'm looking for curved mechanistic arrows and appropriate formal charges on intermediates. OMe MeO OMe Me2N NMe2 OTBS OH xylenes OMe 'OTBSarrow_forwardWhat is the polymer made from the following monomers? What type of polymerization is used for each? а. ОН H2N но b. ن -NH2 d. H₂N NH2 довarrow_forwardCondensation polymers are produced when monomers containing two different functional groups link together with the loss of a small molecule such as H2O. The difunctional monomer H2N(CH2)6COOH forms a condensation polymer. Draw the carbon-skeleton structure of the dimer that forms from this monomer.arrow_forward
- What is the structure of the monomer?arrow_forward→ BINDERIYA GANBO... BINDERIYA GANBO. AP Biology Notes Gamino acid chart - G... 36:22 司 10 ☐ Mark for Review Q 1 Hide 80 8 2 =HA O=A¯ = H₂O Acid HIO HBrO HCIO Question 10 of 35 ^ Σ DELL □ 3 % Λ & 6 7 * ∞ 8 do 5 $ 4 # m 3 ° ( 9 Highlights & Notes AXC Sign out Carrow_forwardWhich representation(s) show polymer structures that are likely to result in rigid, hard materials and those that are likely to result in flexible, stretchable, soft materials?arrow_forward
- 3. Enter the molecular weight of the product obtained from the Williamson Ether Synthesis? OH OH & OH excess CH3l Ag₂Oarrow_forwardPlease answer 1, 2 and 3 on the endarrow_forwardIn the box below, specify which of the given compounds are very soluble in polar aprotic solvents. You may select more than one compound. Choose one or more: NaCl NH4Cl CH3CH2CH2CH2CH2CN CH3CH2OH hexan-2-one NaOH CH3SCH3arrow_forward
- On the following structure, select all of the atoms that could ACCEPT a hydrogen bond. Ignore possible complications of aromaticity. When selecting be sure to click on the center of the atom.arrow_forwardRank the compounds below from lowest to highest melting point.arrow_forward18 Question (1 point) Draw the line structure form of the given partially condensed structure in the box provided. :ÖH HC HC H2 ΙΩ Н2 CH2 CH3 CH3 partially condensed formarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY