
Concept explainers
Interpretation: Three figures of barometers in which the centre one is located at the sea level are shown. The barometer that is most likely to reflect the atmospheric pressure in Denver,
Concept introduction: There occurs variation in pressure from one place to another. Different units are used for representing the pressure. The unit
To determine: The barometer that is most likely to reflect the atmospheric pressure in Denver,
Answer to Problem 6.1VP
Solution
The figure (a) is therefore, the correct option.
Explanation of Solution
Explanation
The graph representing the variation of atmospheric pressure at different locations on the surface of the earth that has relation with the mass of the air in column present above that location is shown as,
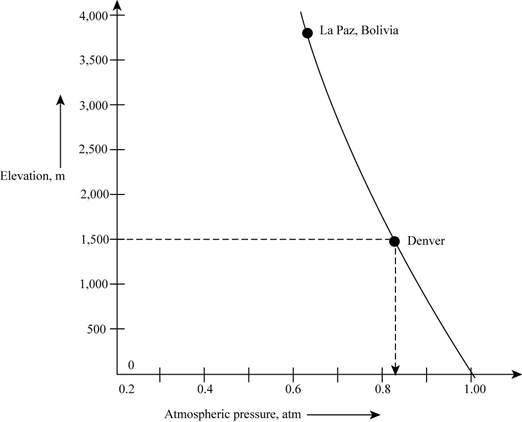
Figure 1
According to the graph, it is observed that with increasing altitude, there occurs a decrease in the mass of the air present above a given area. If mass becomes less it means that the chances of collisions are very less and if the collision will not occur then the force exerted by air will be small.
Therefore, the figure that represents the decrease in column is most likely to reflect the atmospheric pressure in Denver,
Conclusion
The figure that represents the decrease in column is most likely to reflect the atmospheric pressure in Denver,
Want to see more full solutions like this?
Chapter 6 Solutions
Chemistry: The Science in Context (Fifth Edition)
- Hi can you please help me solve this problem? thank youarrow_forwardAn electrode process takes place at a metal-solution interface. Indicate the current condition that must be met for Faradaic rectification to occur.arrow_forwardAt a metal-solution interface, an electron is exchanged, and the symmetry factor beta < 0.5 is found in the Butler-Volmer equation. What does this indicate?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





