
Chemistry: Structure and Properties Custom Edition for Rutgers University General Chemistry
15th Edition
ISBN: 9781269935678
Author: Nivaldo J. Tro
Publisher: Pearson Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 43E
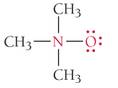
Determine the formal charges of the atoms shown in red
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A solution contains 0.097 M Ce3+, 1.55x10-3 M Ce4+, 1.55x10-3 M Mn2+, 0.097 M MnO4-, and 1.00 M HClO4 (F= 9.649 x 104 C/mol).
a) Write a balanced net reaction that can occur between species in this solution.
b) Calculate deltaG0 and K for the reaction.
c) Calculate E and deltaG for the conditions given.
Ce4+ + e- = Ce3+ E0= 1.70 V
MnO4- + 8H+ + 5e- = Mn2+ + 4H2O E0= 1.507 V
1. Provide a step-by-step mechanism for formation of ALL STEREOISOMERS in
the following reaction.
Na HCO3 (Sodium bicarbonate, baking soda) is not soluble in CH2Cl2. The powder is
a weak base used to neutralize strong acid (pKa < 0) produced by the reaction.
Redraw the product to show the configuration(s) that form at C-2 and C-4.
Br2
OH
CH2Cl2
Na* HCO3
Br
HO
OH
+ Na Br +
2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If
two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each
reagent set. If a reaction cannot be carried out with reagents (sets)
class, write NP (not possible) in the solvent box for reagent set #1.
Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s).
Solvents: CH2Cl2 (A);
H₂O (B);
Reagents:
HBr (1);
R₂BH (6);
H2SO4 (2);
CH3OH (C);
Br₂ (3);
CH3CO₂H (D)
NaHCO3 (4);
Hg(OAc)2 (5);
H₂O2/HO (7);
NaBH4 (8)
Reagent Set #1
Reagent Set #2
FGI
+ enant
OH
Solvent Reagent(s) Solvent Reagent(s)
Chapter 6 Solutions
Chemistry: Structure and Properties Custom Edition for Rutgers University General Chemistry
Ch. 6 - Which set of elements is arranged in order of...Ch. 6 - Prob. 2SAQCh. 6 - Which pair of atoms forms the most polar bond? C...Ch. 6 - Which pair of atoms forms a nonpolar covalent...Ch. 6 - Prob. 5SAQCh. 6 - Prob. 6SAQCh. 6 - Prob. 7SAQCh. 6 - Prob. 8SAQCh. 6 - Prob. 9SAQCh. 6 - Prob. 10SAQ
Ch. 6 - Prob. 11SAQCh. 6 - Predict the relative bond angles in BF3 and SO2Ch. 6 - Predict the molecular geometry about N in the...Ch. 6 - Which molecule is polar?Ch. 6 - What is electronegativity? What are the periodic...Ch. 6 - Explain the difference between a pure covalent...Ch. 6 - What is meant by the percent ionic character of a...Ch. 6 - Prob. 4ECh. 6 - What is the magnitude of the dipole moment formed...Ch. 6 - What is the basic procedure for writing a covalent...Ch. 6 - How do you determine the number of electrons that...Ch. 6 - What are resonance structures? What is a resonance...Ch. 6 - Prob. 9ECh. 6 - Prob. 10ECh. 6 - Prob. 11ECh. 6 - Prob. 12ECh. 6 - What is bond energy?Ch. 6 - Give some examples of some typical bond lengths....Ch. 6 - Why is molecular geometry important? Cite some...Ch. 6 - According to VSEPR theory, what determines the...Ch. 6 - Name and draw the five basic electron geometries,...Ch. 6 - Explain the difference between electron geometry...Ch. 6 - List the correct electron and molecular geometries...Ch. 6 - How do you apply VSEPR theory to predict the shape...Ch. 6 - How do you determine if a molecule is polar?Ch. 6 - Why is polarity a key connection between the...Ch. 6 - Prob. 23ECh. 6 - Determine if a bond between each pair of atoms...Ch. 6 - Prob. 25ECh. 6 - Draw the Lewis structure for BrF with an arrow...Ch. 6 - Prob. 27ECh. 6 - Write the Lewis structure for each molecule. NF3...Ch. 6 - Prob. 29ECh. 6 - Write the Lewis structure for each molecule. CH2O...Ch. 6 - Prob. 31ECh. 6 - Prob. 32ECh. 6 - Write the Lewis structure for each molecule or ion...Ch. 6 - Prob. 34ECh. 6 - Write a Lewis structure that obeys the octet rule...Ch. 6 - Prob. 36ECh. 6 - Use formal charge to determine which Lewis...Ch. 6 - Prob. 38ECh. 6 - How important is this resonance structure to the...Ch. 6 - Prob. 40ECh. 6 - Prob. 41ECh. 6 - Prob. 42ECh. 6 - Determine the formal charges of the atoms shown in...Ch. 6 - Prob. 44ECh. 6 - Prob. 45ECh. 6 - Write the Lewis structure for each molecule (octet...Ch. 6 - Prob. 47ECh. 6 - Write Lewis structures for each molecule or ion....Ch. 6 - Prob. 49ECh. 6 - Write Lewis structures for each molecule or ion....Ch. 6 - List these compounds in order of increasing...Ch. 6 - Which of these compounds has the stronger...Ch. 6 - A molecule with the formula AB3 has a trigonal...Ch. 6 - A molecule with the formula AB3 has a trigonal...Ch. 6 - For each molecular geometry shown here, list the...Ch. 6 - For each molecular geometry shown here, list the...Ch. 6 - Determine the electron geometry, molecular...Ch. 6 - Determine the electron geometry, molecular...Ch. 6 - Which species has the smaller bond angle, H3O+ or...Ch. 6 - Which species has the smaller bond angle; C1O4- or...Ch. 6 - Determine the molecular geometry and draw each...Ch. 6 - Determine the molecular geometry and draw each...Ch. 6 - Determine the molecular geometry about each...Ch. 6 - Prob. 64ECh. 6 - Prob. 65ECh. 6 - Prob. 66ECh. 6 - Prob. 67ECh. 6 - Determine the geometry about each interior atom in...Ch. 6 - Explain why CO2 and CCl4 are both nonpolar even...Ch. 6 - CH3F is a polar molecule, even though the...Ch. 6 - Determine whether each molecule in Exercise 57 is...Ch. 6 - Prob. 72ECh. 6 - Determine whether each molecule or ion is polar or...Ch. 6 - Determine whether each molecule is polar or...Ch. 6 - Each compound contains both ionic and covalent...Ch. 6 - Prob. 76ECh. 6 - Carbon ring structures are common in organic...Ch. 6 - Prob. 78ECh. 6 - Prob. 79ECh. 6 - Diazomethane is a highly poisonous, explosive...Ch. 6 - Prob. 81ECh. 6 - Phosgene (Cl2CO) is a poisonous gas that was used...Ch. 6 - The cyanate ion (OCN-) and the fulminate ion...Ch. 6 - Prob. 84ECh. 6 - Prob. 85ECh. 6 - Prob. 86ECh. 6 - Prob. 87ECh. 6 - Prob. 88ECh. 6 - Prob. 89ECh. 6 - Free radicals are important in many...Ch. 6 - A compound composed of only carbon and hydrogen is...Ch. 6 - A compound composed of only carbon and chlorine is...Ch. 6 - Prob. 93ECh. 6 - The genetic code is based on four different bases...Ch. 6 - Prob. 95ECh. 6 - Prob. 96ECh. 6 - Prob. 97ECh. 6 - A 0.167-g sample of an unknown compound contains...Ch. 6 - Use the dipole moments of HF and HCI (given at the...Ch. 6 - One form of phosphorus exists as P4 molecules....Ch. 6 - A compound has the formula C8H8 and does not...Ch. 6 - Prob. 102ECh. 6 - The bond angles increase steadily in the series...Ch. 6 - Draw the Lewis structure for acetamide (CH3CONH2),...Ch. 6 - Prob. 105ECh. 6 - In the very first chapter of this book, we...Ch. 6 - Which statement best captures the fundamental idea...Ch. 6 - Prob. 108E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Germanium (Ge) is a semiconductor with a bandgap of 2.2 eV. How could you dope Ge to make it a p-type semiconductor with a larger bandgap? Group of answer choices It is impossible to dope Ge and have this result in a larger bandgap. Dope the Ge with silicon (Si) Dope the Ge with gallium (Ga) Dope the Ge with phosphorus (P)arrow_forwardWhich of the following semiconductors would you choose to have photons with the longest possible wavelengths be able to promote electrons to the semiconductor's conduction band? Group of answer choices Si Ge InSb CdSarrow_forwardWhich of the following metals is the only one with all of its bands completely full? Group of answer choices K Na Ca Alarrow_forward
- 2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); Reagents: H₂O (B); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); HBr (1); R₂BH (6); H2SO4 (2); CH3OH (C); Br₂ (3); H₂O₂ / HO- (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI OH - α-α Br + enant Solvent Reagent(s) Solvent Reagent(s)arrow_forwardBased on concepts from Lecture 3-5, which of the following ionic compounds should be most soluble in water? Group of answer choices MgO BeO CaO BaOarrow_forwardFrom an energy standpoint, which two process - in the correct order - are involved in the dissolving of an ionic compound crystal? Group of answer choices Water coordination to the ions followed by sublimation into the gas phase Sublimation of the crystal into gas-phase ions followed by water coordination to the ions Ion dissociation from the crystal followed by water coordination to the ions Water coordination to the ions followed by ion dissociation from the crystalarrow_forward
- For which Group 2 metal (M), is this process the most exothermic? M2+(g) + O2−(g) + CO2(g) → MO(s) + CO2(g) Group of answer choices M = Sr M = Mg M = Ca M = Baarrow_forward2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); H₂O (B); Reagents: HBr (1); H2SO4 (2); CH3OH (C); Br₂ (3); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); R₂BH (6); H₂O₂ / HO- (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI Solvent Reagent(s) Solvent Reagent(s) HO OHarrow_forwardFor which of the following ionic compounds would you expect the smallest difference between its theoretical and experimental lattice enthalpies? (You may assume these all have the same unit cell structure.) Electronegativities: Ca (1.0), Fe (1.8), Mg (1.2), O (3.5), S (2.5), Zn (1.6) Group of answer choices ZnO MgS CaO FeSarrow_forward
- In the Born-Haber cycle for KCl crystal formation, what enthalpy component must be divided by two? Group of answer choices KCl(s) enthalpy of formation Ionization energy for K(g) K(s) sublimation enthalpy Cl2 bond dissociation enthalpyarrow_forward2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); H₂O (B); Reagents: HBr (1); R₂BH (6); H2SO4 (2); CH3OH (C); Br₂ (3); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); H₂O₂ / HO (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI хот Br Solvent Reagent(s) Solvent Reagent(s)arrow_forwardWhat is the correct chemical equation for the lattice formation reaction for CaBr2? Group of answer choices Ca2+(g) + 2 Br−(g) → CaBr2(s) ½ Ca2+(g) + Br−(g) → ½ CaBr2(s) Ca(s) + Br2(l) → CaBr2(s) Ca(s) + 2 Br−(g) → CaBr2(s)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY