
Interpretation:
The sequences of arrow-pushing pattern in the given reactions have to be identified and compared.
Concept Introduction:
Mechanism of the reaction is the step-by-step description of the process by which reactants are changed into products.
There are only four characteristic patterns, and all ionic mechanisms are simply combinations of these four steps, and they are,
- (1) Nucleophilic attack
When we identify a nucleophilic site and an electrophilic site, the arrow in the mechanism step shows the nucleophile attacking.
- (2) Proton transfer
- (3) Loss of leaving group
- (4) Rearrangements
Rearrangements will always occur when an alkyl group or hydrogen can shift to form a more stable carbocation. There are mainly two types of rearrangement shifts and they are,
Curved arrows show the bonds that are formed and the bonds that are broken in a reaction.
Curved arrows used to understand a reaction mechanism.
Curved arrows are drawn to show how the electrons move as new covalent bonds are formed existing covalent bonds are broken.
Each arrow represents the simultaneous movement of two electrons from a nucleophile towards an electrophile.
Nucleophile: It is negatively charged species which seeks for positive charge and hence donate pair of electrons to positively charged species (electrophiles) which results in the formation of
In nucleophilic substitution reaction a nucleophile is the species with an unshared electron pair which reacts with an electron deficient carbon. And so the leaving group is substituted by a nucleophile.
Ipso substitution reaction: It is the one of the
The mechanism of desulfonation curved arrow pattern is given below.
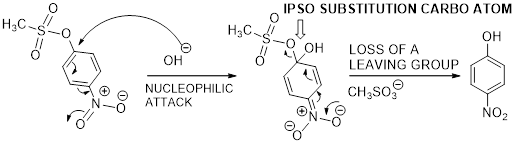
Trending nowThis is a popular solution!

Chapter 6 Solutions
Organic Chemistry 3rd.ed. Klein Evaluation/desk Copy
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





