
In this chapter, we have learned about the
suppose it is hypothesized that it requires more energy to remove an electron from a metal that has atoms with one or more half-filled shells than from those that do not.
- Design a series of experiments involving the photoelectric effect that would test the hypothesis.
- What experimental apparatus would be needed to test the hypothesis? Its not necessary that you name actual equipment but rather that you imagine how the apparatus would work-think in terms of the types of measurements that would be needed, and what capability you would need in your apparatus.
- Describe the type of data you would collect and how you would analyze the data to see whether the hypothesis were correct.
- Could your experiments be extended to test the hypothesis for other parts of the periodic table, such as the lanthanide or actinide elements?
Interpretation: The experiments, apparatus and the type of data to test the given hypothesis is to be determined.
(a) A series of experiment involving the photoelectric effect that would test the hypothesis needs to be designed.
(b) The experimental apparatus required to test the given hypothesis should be determined.
(c)The type of data required to conclude whether the given hypothesis is correct or not should be determined.
(d) If the given hypothesis can be tested on lanthanides or actinides or not should be identified.
Concept Introduction: The light falls on the surface of the metal and results in the ejection of electron form its surface. This process is known as the photoelectric effect.
Answer to Problem 1DE
Solution: (a) When the light incident on the metal plate having one or more half-filled orbital; one does not observe the collection of electrons on the collector plate. But as the energy increases the electrons started collecting on the collector plate. This proves the given hypothesis.
(b) The experimental apparatus to study the photoelectric effect consists of the metal plate, collector plate, battery and voltmeter.
(c) The plot of stopping potential as a function of frequency is used to conclude that the given hypothesis is correct.
(d) The given hypothesis is not applicable on lanthanides and actinides because of the presence of d and f orbitals.
(a)
Explanation of Solution
The hypothesis says that that it requires more energy to remove an electron from the metal that has one or more half filled shells.
The ejection of electrons from the metal surface is tested by using the photoelectric effect by considering the apparatus consists of the metal plate on which the light is incident and the collector plate on which the electrons get collected. These two plates are connected to the electric circuit consists of battery, photodiode with amplifier and the voltmeter with reverse voltage.
When the light incident on the metal plate having one or more half filled orbital; one does not observed the collection of electrons on the collector plate. But as the energy increases the electrons starting collecting on the collector plate. This proves the given hypothesis.
(b)
Explanation:
The apparatus to study the photoelectric effect has the following parts,
- A photodiode with an amplifier.
- A digital voltmeter with reverse voltage.
- Batteries to operate amplifier and to provide reverse voltage.
- A monochromatic light source.
- The incident light beam intensity must adjust using a neutral filter.
The apparatus for testing the given hypothesis is shown below:
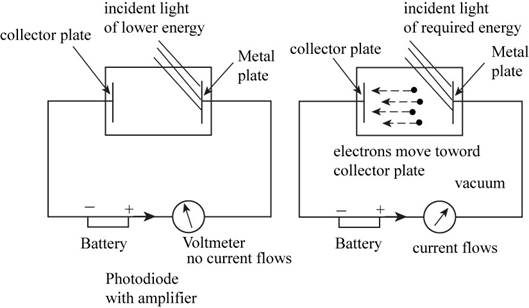
Figure 1
(c)
Explanation:
The data of frequency and wave length of different light source is collected and it is used in the apparatus of photoelectric effect. The different percentage transmission values as the function of intensity will be observed. The plot of stopping potential as a function of frequency will be observed from this data which conclude whether the hypothesis is correct or not.
(d)
Explanation:
The given hypothesis says that more energy is required to eject the electron form a metal having half filled orbitals as compared to those who have not. But the orbital of lanthanides and actinides are diffused in nature and they are larger in size. Therefore, they can easily accept and eject electrons by using lower energy radiation. Therefore, the given hypothesis cannot be tested on the lanthanides and actinides.
- The ejection of electrons is tested by using the apparatus consists of metal plate and collector plate.
- The experimental apparatus to study the photoelectric effect consists of the metal plate, collector plate, battery and voltmeter.
- The plot of stopping potential as a function of frequency is used to conclude that the given hypothesis is correct.
- The given hypothesis is not applicable on lanthanides and actinides because of the presence of d and f orbitals.
Want to see more full solutions like this?
Chapter 6 Solutions
Chemistry: The Central Science (14th Edition)
Additional Science Textbook Solutions
Organic Chemistry (8th Edition)
Human Biology: Concepts and Current Issues (8th Edition)
Campbell Essential Biology with Physiology (5th Edition)
Campbell Biology (11th Edition)
Living By Chemistry: First Edition Textbook
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
- Show how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forwardHelp me solve this problem. Thank you in advance.arrow_forward22.7 Predict the monoalkylated products of the following reactions with benzene. (a) AlCl3 Ya (b) AlCl3 (c) H3PO4 (d) 22.8 Think-Pair-Share AICI3 The reaction below is a common electrophilic aromatic substitution. SO3 H₂SO4 SO₂H (a) Draw the reaction mechanism for this reaction using HSO,+ as the electrophile. (b) Sketch the reaction coordinate diagram, where the product is lower in energy than the starting reactant. (c) Which step in the reaction mechanism is highest in energy? Explain. (d) Which of the following reaction conditions could be used in an electrophilic aro- matic substitution with benzene to provide substituted phenyl derivatives? (i) AICI3 HNO3 H₂SO4 K2Cr2O7 (iii) H₂SO4 (iv) H₂PO₁arrow_forward
- Is an acid-base reaction the only type of reaction that would cause leavening products to rise?arrow_forwardHelp me understand this! Thank you in advance.arrow_forward22.22 For each compound, indicate which group on the ring is more strongly activating and then draw a structural formula of the major product formed by nitration of the compound. Br CHO (a) CH3 (b) (c) CHO CH3 SO₂H (d) ☑ OCHS NO₂ (e) (f) CO₂H NHCOCH3 NHCOCH, (h) CHS 22.23 The following molecules each contain two aromatic rings. (b) 000-100- H3C (a) (c) Which ring in each undergoes electrophilic aromatic substitution more readily? Draw the major product formed on nitration.arrow_forward
- V Consider this step in a radical reaction: Br: ? What type of step is this? Check all that apply. Draw the products of the step on the right-hand side of the drawing area below. If more than one set of products is possible, draw any set. Also, draw the mechanism arrows on the left-hand side of the drawing area to show how this happens. ⚫ionization termination initialization neutralization none of the abc Explanation Check 80 Ο F3 F1 F2 2 F4 01 % do5 $ 94 #3 X 5 C MacBook Air 25 F5 F6 66 ©2025 ˇ F7 29 & 7 8arrow_forwardShow how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forwardno aiarrow_forward
- Polymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts.arrow_forwardDraw a tetramer if this alternating copolymer pleasearrow_forwardDraw the monomers required to synthesize this condensation polymer.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





