
Concept explainers
(a)
To determine: The value of Vmax and Km of enzyme prostaglandin endoperoxide synthase.
Introduction:
Prostaglandin is class of lipid which is present at a site of injury and tissue damage in body. It is involved in healing process and induces inflammation, and initiation of pain.
(a)
Explanation of Solution
Pictorial representation:
Table 1 shows the rate of formation of prostaglandin from arachidonic acid and Fig.1 shows the Lineweaver Burk plot, a double reciprocal plotting for 1/V0 Vs. 1/[S].
Table 1
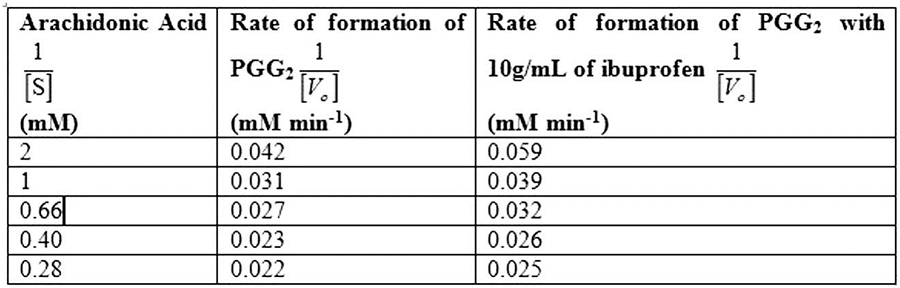
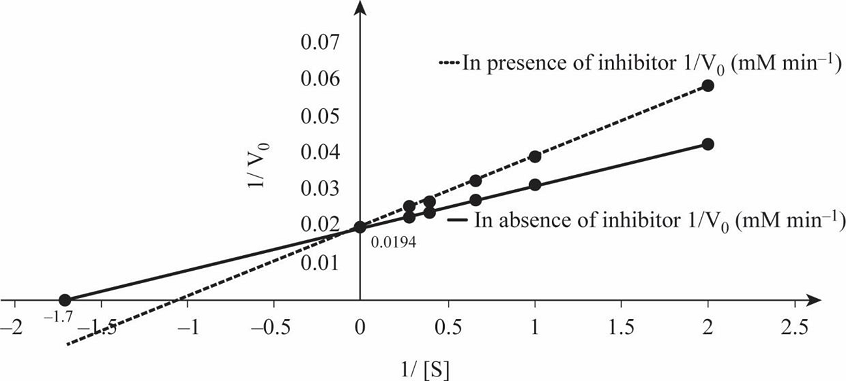
Fig.1: Lineweaver Burk plot.
Lineweaver-Burk equation is the reciprocal of Michaelis-Menten equation, and given as:
Michaelis-Menten equation
Lineweaver-Burk equation
So, by reciprocating the values given in first and second columns of the given table, we get 1/[S] and 1/V0 values in absence of the inhibitor, as mentioned in Table 1. By plotting these values we obtain the Lineweaver-Burk graph depicted in Fig.1. From the graph, calculating the V max in the absence of inhibitor:
Calculating the Km in the absence of inhibitor:
The Vmax of enzyme in the absence of inhibitor is 51.5mM/min, while Km of enzyme in the absence of inhibitor is 0.59mM.
(b)
To determine: The type of inhibition that ibuprofen exerts on prostaglandin endoperoxide synthase.
Introduction:
Enzyme inhibitors are defined as chemical molecules that bind at active site of enzymes and prevent the binding of substrate with enzyme. There are two types of inhibitors such as reversible and irreversible inhibitors.
(b)
Explanation of Solution
Lineweaver-Burk equation is the reciprocal of Michaelis-Menten equation is given as:
Michaelis-Menten equation
Lineweaver-Burk equation
So, by reciprocating the given values in column first and third, we get the rate of formation of prostaglandin from arachidonic acid in presence of inhibitor ibuprofen, as mentioned in Table 1. By plotting these values we obtain the Lineweaver-Burk graph for 1/[S] and 1/V0 in presence of inhibitor, as depicted in Fig.1.
Calculating the Vmax in presence of inhibitor:
Vmax in the presence of inhibitor:
Km of enzyme in the presence of inhibitor:
The Vmax of enzyme both in the presence and absence of inhibitor is 51.54 mM/min, while Km of enzyme in the presence and absence of inhibitor is 0.83mM and 0.59mM.
Prostaglandin is involved in initiation of pain, and it is synthesized by prostaglandin endoperoxide synthase. Ibuprofen inhibits the activity of this enzyme by binding at the active site and preventing binding of substrate with enzyme. The double reciprocal graph in Fig.1 shows when competitive inhibitor ibuprofen is present, the Vmax remains unchanged while Km increases. Thus, -1/Km value is closer to the origin in the graph depicted in Fig.1. In competitive inhibition, Vmax remains unchanged while Km increases. Therefore, ibuprofen is a competitive inhibitor of prostaglandin.
The inhibition of prostaglandin by ibuprofen is example of competitive inhibition.
Want to see more full solutions like this?
Chapter 6 Solutions
SAPLINGPLUS FOR PRINCIPLES OF BIOCHEMIS
- Please help determine the standard curve for my Kinase Activity in Excel Spreadsheet. Link: https://mnscu-my.sharepoint.com/personal/vi2163ss_go_minnstate_edu/_layouts/15/Doc.aspx?sourcedoc=%7B958f5aee-aabd-45d7-9f7e-380002892ee0%7D&action=default&slrid=9b178ea1-b025-8000-6e3f-1cbfb0aaef90&originalPath=aHR0cHM6Ly9tbnNjdS1teS5zaGFyZXBvaW50LmNvbS86eDovZy9wZXJzb25hbC92aTIxNjNzc19nb19taW5uc3RhdGVfZWR1L0VlNWFqNVc5cXRkRm4zNDRBQUtKTHVBQldtcEtWSUdNVmtJMkoxQzl3dmtPVlE_cnRpbWU9eEE2X291ZHIzVWc&CID=e2126631-9922-4cc5-b5d3-54c7007a756f&_SRM=0:G:93 Determine the amount of VRK1 is present 1. Average the data and calculate the mean absorbance for each concentration/dilution (Please over look for Corrections) 2. Blank Correction à Subtract 0 ug/mL blank absorbance from all readings (Please over look for Corrections) 3. Plot the Standard Curve (Please over look for Corrections) 4. Convert VRK1 concentration from ug/mL to g/L 5. Use the molar mass of VRK1 to convert to M and uM…arrow_forwardMacmillan Learning Cholesterol synthesis begins with the formation of mevalonate from acetyl CoA. This process activates mevalonate and converts it to isopentenyl pyrophosphate. Identify the atoms in mevalonate and isopentenyl pyrophosphate that will be labeled from acetyl CoA labeled with 14C in the carbonyl carbon. Place 14C atoms and C atoms to denote which carbon atoms are labeled and which are not labeled. H₂C COA 14C-labeled acetyl-CoA HHH [c] H H OH 014C - OH H HH H Mevalonate CH3 H H 14C H Η H H Incorrect Answer of o -P-O-P-0- Isopentenyl pyrophosphate с Answer Bank 14Carrow_forwardDraw the reaction between sphingosine and arachidonic acid. Draw out the full structures.arrow_forward
- Draw both cis and trans oleic acid. Explain why cis-oleic acid has a melting point of 13.4°C and trans-oleic acid has a melting point of 44.5°C.arrow_forwardDraw the full structure of the mixed triacylglycerol formed by the reaction of glycerol and the fatty acids arachidic, lauric and trans-palmitoleic. Draw the line structure.arrow_forwardDraw out the structure for lycopene and label each isoprene unit. "Where is lycopene found in nature and what health benefits does it provide?arrow_forward
- What does it mean to be an essential fatty acid? What are the essential fatty acids?arrow_forwardCompare and contrast primary and secondary active transport mechanisms in terms of energy utilisation and efficiency. Provide examples of each and discuss their physiological significance in maintaining ionic balance and nutrient uptake. Rubric Understanding the key concepts (clearly and accurately explains primary and secondary active transport mechanisms, showing a deep understanding of their roles) Energy utilisation analysis ( thoroughly compares energy utilisation in primary and secondary transport with specific and relevant examples Efficiency discussion Use of examples (provides relevant and accurate examples (e.g sodium potassium pump, SGLT1) with clear links to physiological significance. Clarity and structure (presents ideas logically and cohesively with clear organisation and smooth transition between sections)arrow_forward9. Which one of the compounds below is the major organic product obtained from the following reaction sequence, starting with ethyl acetoacetate? 요요. 1. NaOCH2CH3 CH3CH2OH 1. NaOH, H₂O 2. H3O+ 3. A OCH2CH3 2. ethyl acetoacetate ii A 3. H3O+ OH B C D Earrow_forward
- 7. Only one of the following ketones cannot be made via an acetoacetic ester synthesis. Which one is it? Ph کہ A B C D Earrow_forward2. Which one is the major organic product obtained from the following reaction sequence? HO A OH 1. NaOEt, EtOH 1. LiAlH4 EtO OEt 2. H3O+ 2. H3O+ OH B OH OH C -OH HO -OH OH D E .CO₂Etarrow_forwardwhat is a protein that contains a b-sheet and how does the secondary structure contributes to the overall function of the protein.arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





