
Concept explainers
(a)
To determine: The value of Vmax and Km of enzyme prostaglandin endoperoxide synthase.
Introduction:
Prostaglandin is class of lipid which is present at a site of injury and tissue damage in body. It is involved in healing process and induces inflammation, and initiation of pain.
(a)
Explanation of Solution
Pictorial representation:
Table 1 shows the rate of formation of prostaglandin from arachidonic acid and Fig.1 shows the Lineweaver Burk plot, a double reciprocal plotting for 1/V0 Vs. 1/[S].
Table 1
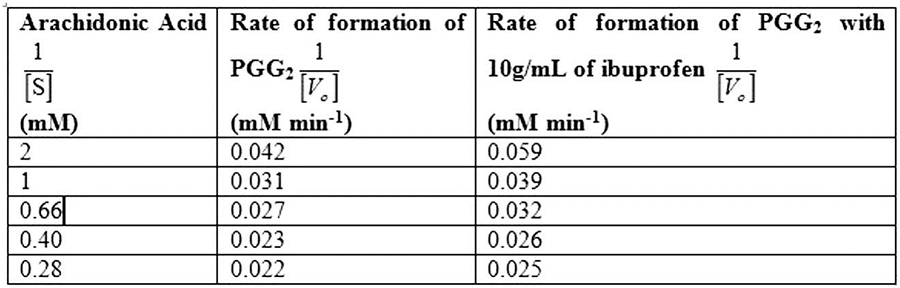
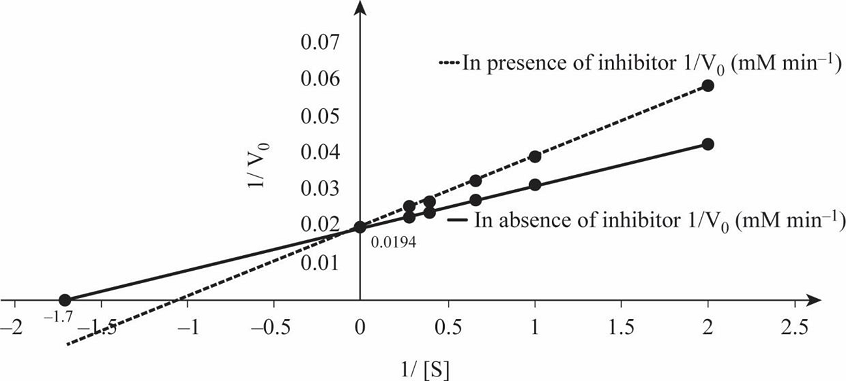
Fig.1: Lineweaver Burk plot.
Lineweaver-Burk equation is the reciprocal of Michaelis-Menten equation, and given as:
Michaelis-Menten equation
Lineweaver-Burk equation
So, by reciprocating the values given in first and second columns of the given table, we get 1/[S] and 1/V0 values in absence of the inhibitor, as mentioned in Table 1. By plotting these values we obtain the Lineweaver-Burk graph depicted in Fig.1. From the graph, calculating the V max in the absence of inhibitor:
Calculating the Km in the absence of inhibitor:
The Vmax of enzyme in the absence of inhibitor is 51.5mM/min, while Km of enzyme in the absence of inhibitor is 0.59mM.
(b)
To determine: The type of inhibition that ibuprofen exerts on prostaglandin endoperoxide synthase.
Introduction:
Enzyme inhibitors are defined as chemical molecules that bind at active site of enzymes and prevent the binding of substrate with enzyme. There are two types of inhibitors such as reversible and irreversible inhibitors.
(b)
Explanation of Solution
Lineweaver-Burk equation is the reciprocal of Michaelis-Menten equation is given as:
Michaelis-Menten equation
Lineweaver-Burk equation
So, by reciprocating the given values in column first and third, we get the rate of formation of prostaglandin from arachidonic acid in presence of inhibitor ibuprofen, as mentioned in Table 1. By plotting these values we obtain the Lineweaver-Burk graph for 1/[S] and 1/V0 in presence of inhibitor, as depicted in Fig.1.
Calculating the Vmax in presence of inhibitor:
Vmax in the presence of inhibitor:
Km of enzyme in the presence of inhibitor:
The Vmax of enzyme both in the presence and absence of inhibitor is 51.54 mM/min, while Km of enzyme in the presence and absence of inhibitor is 0.83mM and 0.59mM.
Prostaglandin is involved in initiation of pain, and it is synthesized by prostaglandin endoperoxide synthase. Ibuprofen inhibits the activity of this enzyme by binding at the active site and preventing binding of substrate with enzyme. The double reciprocal graph in Fig.1 shows when competitive inhibitor ibuprofen is present, the Vmax remains unchanged while Km increases. Thus, -1/Km value is closer to the origin in the graph depicted in Fig.1. In competitive inhibition, Vmax remains unchanged while Km increases. Therefore, ibuprofen is a competitive inhibitor of prostaglandin.
The inhibition of prostaglandin by ibuprofen is example of competitive inhibition.
Want to see more full solutions like this?
Chapter 6 Solutions
SaplingPlus for Lehninger Principles of Biochemistry (Six-Month Access)
- The two half reactions for beginning and end of the electron transport chain are given below in standard form. Calculate & for the overall process. Using the Nernst equation (AG° = -n Fo, F= 96.485 kJ/volt mol), calculate AG°. Explain the need for a stepwise process in the electron transport chain. NAD* + H+ + 2 e- = NADH ½ 0г + 2H+ + 2е- = H20 = -0.32v E = +0.82Varrow_forwardanswer the questions and the example steps should be from carbohydrates glycolysis and citric acid cycle. Please put down reactions and structuresarrow_forwardidentify the general type of reaction catalyzed and an example step from glycolisis structure for each of the following enzymes/ co factor Kinase, isomerase, mutase, dehydrogenase, NAD+ , FADarrow_forward
- fill in the blanks with the missing structures and give namesarrow_forwardfill in the table and identify the general type of reaction catalayzed and an example step from the structures in the second page so you will answer the questions from the first page the second one is just a reference urgently!arrow_forwardPlease draw out the molecular structures of each molecule and show how each enzyme + cofactor would affect the following molecule in the human metabolic pathway. (This is a metabolic map)arrow_forward
- Please draw out the molecular structures of each molecule and show how an enzyme + cofactor would affect the following molecule in the human metabolic pathway to create energy.arrow_forwardPlease draw out the molecular structures of each molecule and show how each enzyme + cofactor would affect the following molecule in the human metabolic pathway.arrow_forwardPlease draw out the mechanism with curved arrows showing electron flow. Pyruvate is accepted into the TCA cycle by a “feeder” reaction using the pyruvate dehydrogenase complex, resulting in acetyl-CoA and CO2. Provide the mechanism for this reaction utilizing the TPP cofactor. Include the roles of all cofactors.arrow_forward
- Pyruvate is accepted into the TCA cycle by a “feeder” reaction using the pyruvate dehydrogenase complex, resulting in acetyl-CoA and CO2. Provide the mechanism for this reaction utilizing the TPP cofactor. Include the roles of all cofactors.arrow_forwardThe mitochondrial ATP synthase has 10 copies of the F0 subunit “c”, and the [H ] in the mitochondrial inner membrane space (IMS) is 6.31 x 10-8 M and the [H + ] in the matrix is 3.16 x 10-9 M. Calculate the minimum membrane potential (∆Ψ) necessary to make ATP synthesis thermodynamically favorable. [Assume ∆G' ofphosphate hydrolysis of ATP is - 45 kJ/mol.]arrow_forwardB- Vitamins are converted readily into important metabolic cofactors. Deficiency in any one of them has serious side effects. a. The disease beriberi results from a vitamin B 1 (Thiamine) deficiency and is characterized by cardiac and neurological symptoms. One key diagnostic for this disease is an increased level of pyruvate and α-ketoglutarate in the bloodstream. How does this vitamin deficiency lead to increased serumlevels of these factors? b. What would you expect the effect on the TCA intermediates for a patient suffering from vitamin B 5 deficiency? c. What would you expect the effect on the TCA intermediates for a patientsuffering from vitamin B 2 /B 3 deficiency?arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





