
Concept explainers
All molecules undergo vibrational motions.
Where n is a quantum number
Interpretation:
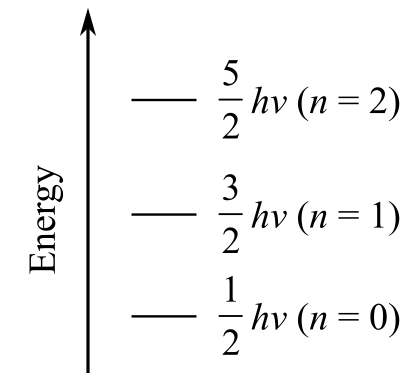
The first three vibrational energy levels for
l is to be drawn. The energy required for the transition of molecule from ground state to first excited state is to be determined, and “the reason for lowest vibrational energy in ground state is not zero, but it is equivalent to
” is to be justified by using Heisenberg Principle.
Concept introduction:
The energy of a photon can be expressed as follows:
Here, E is the energy of photon,
Heisenberg uncertainty principle explains that the product of uncertainty in position and momentum of particle cannot be less than
Here,
denotes uncertainty in position,
denotes uncertainty in momentum, and
denotes Planck’s constant
Answer to Problem 112AP
Solution:
a)
(b)
(c) Consider the diatomic molecule
This is disallowed by the Heisenberg uncertainty principle.
Explanation of Solution
a) Plot the first three vibrational energy levels for HCl
The vibrational energy of diatomic molecules is given as
The first three vibrational energy levels n are
For
For
For
The vibrational energy for the first three energy levels is

b) The vibrational energy required to excite HCl molecule from ground state to first exited state.
The vibrational energy for the ground state and first excited state are as follows:
For the transition from ground state
Now, substitute the value
and
in the above equation
c) Justify the prediction “that the lowest vibrational energy in the ground state is not zero, but it is equivalent to
Consider the diatomic molecule
As the two atoms are bonded to each other, the uncertainty in position, that is,
Thus,
So, this is disallowed by the Heisenberg uncertainty principle.
Want to see more full solutions like this?
Chapter 6 Solutions
BURDGE CHEMISTRY VALUE ED (LL)
Additional Science Textbook Solutions
Biology: Life on Earth with Physiology (11th Edition)
Human Biology: Concepts and Current Issues (8th Edition)
Physical Science
Fundamentals Of Thermodynamics
Organic Chemistry
Loose Leaf For Integrated Principles Of Zoology
- H2SO4 (cat.), H₂O 100 °C NH₂arrow_forwardX Draw the major products of the elimination reaction below. If elimination would not occur at a significant rate, check the box under the drawing area instead. ది www. Cl + OH Elimination will not occur at a significant rate. Click and drag to start drawing a structure.arrow_forwardNonearrow_forward
- 1A H 2A Li Be Use the References to access important values if needed for this question. 8A 3A 4A 5A 6A 7A He B C N O F Ne Na Mg 3B 4B 5B 6B 7B 8B-1B 2B Al Si P 1B 2B Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe * Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Rf Ha ****** Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Analyze the following reaction by looking at the electron configurations given below each box. Put a number and a symbol in each box to show the number and kind of the corresponding atom or ion. Use the smallest integers possible. cation anion + + Shell 1: 2 Shell 2: 8 Shell 3: 1 Shell 1 : 2 Shell 2 : 6 Shell 1 : 2 Shell 2: 8 Shell 1: 2 Shell 2: 8arrow_forwardNonearrow_forwardIV. Show the detailed synthesis strategy for the following compounds. a. CH3CH2CH2CH2Br CH3CH2CCH2CH2CH3arrow_forward
- Do the electrons on the OH participate in resonance with the ring through a p orbital? How many pi electrons are in the ring, 4 (from the two double bonds) or 6 (including the electrons on the O)?arrow_forwardPredict and draw the product of the following organic reaction:arrow_forwardNonearrow_forward
- Redraw the molecule below as a skeletal ("line") structure. Be sure to use wedge and dash bonds if necessary to accurately represent the direction of the bonds to ring substituents. Cl. Br Click and drag to start drawing a structure. : ☐ ☑ Parrow_forwardK m Choose the best reagents to complete the following reaction. L ZI 0 Problem 4 of 11 A 1. NaOH 2. CH3CH2CH2NH2 1. HCI B OH 2. CH3CH2CH2NH2 DII F1 F2 F3 F4 F5 A F6 C CH3CH2CH2NH2 1. SOCl2 D 2. CH3CH2CH2NH2 1. CH3CH2CH2NH2 E 2. SOCl2 Done PrtScn Home End FA FQ 510 * PgUp M Submit PgDn F11arrow_forwardNonearrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning



