
ORGANIC CHEM W/BIOLOGICAL TOP. ACCESS
6th Edition
ISBN: 9781264382545
Author: SMITH
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5.7, Problem 21P
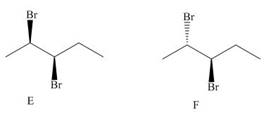
Compounds E and F are two isomers of 2, 3-dibromopentane drawn in staggered conformations.
Which compounds (A-D) in Figure 5.8 are identical to E and F?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
"Water gas" is an industrial fuel composed of a mixture of carbon monoxide and hydrogen gases. When this
fuel is burned, carbon dioxide and water result. From the information given below, write a balanced equation
and determine the enthalpy of this reaction:
CO(g) + O2(g) → CO₂(g) + 282.8 kJ
H2(g) + O2(g) → H₂O(g) + 241.8 kJ
MacBook Air
Page of 3
4. Calculate AG for the following reaction at 25°C. Will the reaction occur (be spontaneous)? How do you
know?
NH3(g) + HCl(g) → NH4Cl(s)
AH=-176.0 kJ
AS-284.8 J-K-1
true or false
The equilibrium constant for this reaction is 0.20.
N2O4(g) ⇔ 2NO2(g)
Based on the above, the equilibrium constant for the following reaction is 5.
4NO2(g) ⇔ 2N2O4(g)
Chapter 5 Solutions
ORGANIC CHEM W/BIOLOGICAL TOP. ACCESS
Ch. 5.1 - Prob. 1PCh. 5.2 - Prob. 2PCh. 5.3 - Draw the mirror image of each compound. Label each...Ch. 5.3 - Prob. 4PCh. 5.3 - A molecule is achiral if it has a plane of...Ch. 5.6 - Prob. 17PCh. 5.6 - Prob. 18PCh. 5.7 - Prob. 19PCh. 5.7 - Prob. 20PCh. 5.7 - Problem 5.18 Compounds E and F are two isomers of...
Ch. 5.8 - Prob. 22PCh. 5.8 - Prob. 23PCh. 5.8 - Prob. 24PCh. 5.9 - Prob. 25PCh. 5.9 - Prob. 26PCh. 5.9 - Prob. 27PCh. 5.10 - Which of the following cyclic molecules are meso...Ch. 5.10 - Prob. 29PCh. 5.11 - Prob. 30PCh. 5.12 - Problem 5.28 The amino acid has the physical...Ch. 5.12 - Prob. 32PCh. 5.12 - Prob. 33PCh. 5.12 - Prob. 34PCh. 5.12 - Prob. 35PCh. 5 - Prob. 39PCh. 5 - Prob. 40PCh. 5 - Prob. 41PCh. 5 - Prob. 42PCh. 5 - 5.40 Determine if each compound is identical to or...Ch. 5 - Prob. 44PCh. 5 - Prob. 45PCh. 5 - Prob. 46PCh. 5 - Prob. 47PCh. 5 - Prob. 48PCh. 5 - Prob. 52PCh. 5 - Prob. 56PCh. 5 - Prob. 61PCh. 5 - Prob. 67PCh. 5 - Prob. 68PCh. 5 - Prob. 69PCh. 5 -
5.67 Artemisinin and mefloquine are widely used...Ch. 5 - 5.68 Saquinavir (trade name Invirase) is a...Ch. 5 - Prob. 72P
Additional Science Textbook Solutions
Find more solutions based on key concepts
1. Rub your hands together vigorously. What happens? Discuss the energy transfers and transformations that take...
College Physics: A Strategic Approach (3rd Edition)
An aluminum calorimeter with a mass of 100 g contains 250 g of water. The calorimeter and water are in thermal ...
Physics for Scientists and Engineers
Describe Mendels conclusions about how traits are passed from generation to generation.
Concepts of Genetics (12th Edition)
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
1. Genetics affects many aspects of our lives. Identify three ways genetics affects your life or the life of a ...
Genetic Analysis: An Integrated Approach (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- true or false The equilibrium constant for this reaction is 0.20. N2O4(g) ⇔ 2NO2(g) Based on the above, the equilibrium constant for the following reaction is 0.4. 2N2O4(g) ⇔ 4NO2(g)arrow_forwardtrue or false Using the following equilibrium, if heat is added the equilibrium will shift toward the reactants. N2(g) + 3H2(g) ⇔ 2NH3(g) + heatarrow_forwardTrue or False Using the following equilibrium, if heat is added the equilibrium will shift toward the products. N2O4(g) + heat ⇔ 2NO2(g)arrow_forward
- true or false Using the following equilibrium, if solid carbon is added the equilibrium will shift toward the products. C(s) + CO2(g) ⇔ 2CO(g)arrow_forwardProvide the complete mechanism for the reaction below. You must include appropriate arrows,intermediates, and formal charges. Please also provide a reason to explain why the 1,4-adduct is preferred over the 1,3-adduct.arrow_forwardWhich of the following pairs are resonance structures of one another? I. III. || III IV + II. :0: n P !༠ IV. EN: Narrow_forward
- Predict the major organic product(s) and byproducts (either organic or inorganic) for thefollowing reactions.arrow_forwardA 8.25 g sample of aluminum at 55°C released 2500 J of heat. The specific heat of aluminum is 0.900 J/g°C. The density of aluminum is 2.70 g/mL. Calculate the final temperature of the aluminum sample in °C.arrow_forwardPredict the major organic product(s) and byproducts (either organic or inorganic) for thefollowing reactions.arrow_forward
- Predict the major organic product(s) and byproducts (either organic or inorganic) for thefollowing reaction.arrow_forwardplease helparrow_forwardExperiment 1 Data Table 1: Conservation of Mass - Initial Mass Data Table 1 Data Table 2 Data Table 3 Data Table 4 Panel 1 Photo 1 Data Table 5 Reaction Mass of test tube and 5.0% HC₂H₂O2 (g) # (A) (B) Mass of NaHCO, (g) Mass of balloon and NaHCO, (g) (C) 0.10 1 0829 14.38g 0.20 2 0.929 14.29g 0.35 1.00g 3 14.25g 0.50 1.14g 14.29 Experiment 1 Data Table 2: Moles of HC2H3O2 Reaction Volume of Mass of Moles of HC₂H₂O₂ 5.0% Vinegar (g) (ML) 5.0 0.25 0042 mol 2 5.0 0.25 0042 mol 3 5.0 0.25 0042 mol 5.0 0.25 0042 mol Experiment 1 Data Table 3: Moles of NaHCO3 Reaction Mass of NaHCO (g) 10g 20g 35g 50g Experiment 1 Data Table 4: Theoretical Yield of CO₂ Reaction # 1 2 3 Experiment 1 Total mass before reaction (g) (D=A+C) 15.29 15.21g 15.25g 15.349 Exercise 1 Data Table 1 Data Table 2 Data Table 3 Data Table 4 Panel 1 Photo 1 Data Table 5 Exercise 1- Data Table 1 Data Table 2 DataTable 3 Data Table 4 Panel 1 Photo 1 Data Table 5 Exercise 1- Moles of NaHCO 0012 mol 0025 mol 0044 mol 0062 mol…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License