
EBK INORGANIC CHEMISTRY
5th Edition
ISBN: 9780133558944
Author: Tarr
Publisher: PEARSON CUSTOM PUB.(CONSIGNMENT)
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 5.2, Problem 5.2E
Interpretation Introduction
Interpretation: Molecular orbitals in figure 5.3(a) should be labeled as g or u.
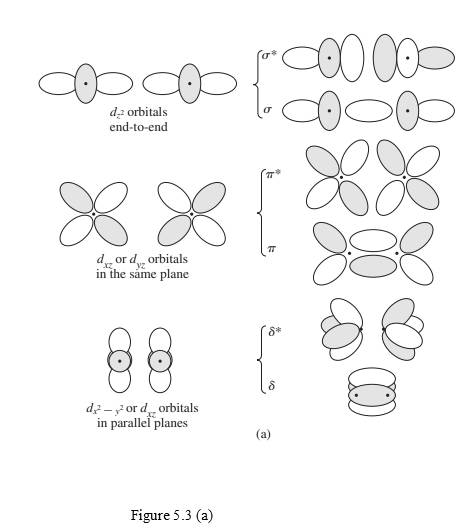
Concept Introduction:
“gerade” (g) used to named orbitals which are symmetric to inversion.
“ungerade” (u) used to named orbitals which are anti-symmetric to inversion.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Is this aromatic?
CHEM2323
E
Tt
PS CH03
Draw and name all monobromo derivatives of pentane, C5H11Br.
Problem 3-33
Name:
Draw structures for the following:
(a) 2-Methylheptane
(d) 2,4,4-Trimethylheptane
Problem 3-35
(b) 4-Ethyl-2,2-dimethylhexane
(e) 3,3-Diethyl-2,5-dimethylnonane
(c) 4-Ethyl-3,4-dimethyloctane
2
(f) 4-Isopropyl-3-methylheptane
KNIE>
Problem 3-42
Consider 2-methylbutane (isopentane). Sighting along the C2-C3 bond:
(a) Draw a Newman projection of the most stable
conformation.
(b) Draw a Newman projection of the least stable
conformation.
Problem 3-44
Construct a qualitative potential-energy diagram for rotation about the C-C bond of 1,2-dibromoethane.
Which conformation would you expect to be most stable? Label the anti and gauche conformations of 1,2-
dibromoethane.
Problem 3-45
Which conformation of 1,2-dibromoethane (Problem 3-44) would you expect to have the largest dipole
moment? The observed dipole moment of 1,2-dibromoethane is µ = 1.0 D. What does this tell you about the
actual conformation of the molecule?
Chapter 5 Solutions
EBK INORGANIC CHEMISTRY
Ch. 5.1 - Repeat the process in the preceding example for...Ch. 5.2 - Prob. 5.2ECh. 5.3 - Use a similar approach to the discussion of HF to...Ch. 5.4 - Sketch the energy levels and the molecular...Ch. 5.4 - Using the D2h character table shown, verify that...Ch. 5.4 - Using orbital potential energies, show that group...Ch. 5.4 - Prob. 5.7ECh. 5.4 - Prob. 5.8ECh. 5.4 - Prob. 5.9ECh. 5.4 - Use the projection operator method to derive...
Ch. 5.4 - Determine the types of hybrid orbitals that are...Ch. 5.4 - Determine the reducible representation for all the...Ch. 5 - Expand the list of orbitais considered in Figures...Ch. 5 - On the basis of molecular orbitals, predict the...Ch. 5 - On the basis of molecular orbitals, predict the...Ch. 5 - Compare the bonding in O22,O2 and O2 Include Lewis...Ch. 5 - Although the peroxide ion, O22 and the acetylide...Ch. 5 - High-resolution photoelectron spectroscopy has...Ch. 5 - a. Prepare a molecular orbital energy-level...Ch. 5 - a. Prepare a molecular orbital energy-level...Ch. 5 - NF is a known molecule a. Construct a molecular...Ch. 5 - The hypofluorite ion, OF can be observed only with...Ch. 5 - Prob. 5.11PCh. 5 - Although KrF+ and XeF+ have been studied, KrBr+...Ch. 5 - Prepare a molecular orbital energy level diagram...Ch. 5 - Methylene, CH2 plays an important role in many...Ch. 5 - Beryllium hydride, BeH2 is linear in the gas...Ch. 5 - In the gas phase, BeF2 forms linear monomeric...Ch. 5 - For the compound XeF2 do the following: a. Sketch...Ch. 5 - TaH5 has been predicted to have C4v symmetry, with...Ch. 5 - Describe the bonding in ozone, o3 on the basis of...Ch. 5 - Describe the bonding in SO3 by using group theory...Ch. 5 - The ion H3+ has been observed, but its structure...Ch. 5 - Use molecular orbital arguments to explain the...Ch. 5 - Prob. 5.23PCh. 5 - Prob. 5.24PCh. 5 - The isomenc ions NSO (thiazate) and SNO...Ch. 5 - Apply the projection operator method to derive the...Ch. 5 - Apply the projection operator method to derive the...Ch. 5 - A set of four group orbitals derived from four 3s...Ch. 5 - The projection operator method has applications...Ch. 5 - Although the cl2+ ion has not been isolated, it...Ch. 5 - BF3 is often described as a molecule in which...Ch. 5 - SF4 has C2v symmetry. Predict the possible...Ch. 5 - Consider a square pyramidal AB5 molecule. Using...Ch. 5 - Prob. 5.34PCh. 5 - For the molecule PCl5 : a. Using the character...Ch. 5 - Molecular modeling software is typically capable...Ch. 5 - Prob. 5.39PCh. 5 - Calculate and display the orbitals for the linear...Ch. 5 - Prob. 5.41PCh. 5 - Prob. 5.42PCh. 5 - Prob. 5.43PCh. 5 - Diborane, B2H6 , has the structure shown. a. Using...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Gas Law Studies 1. Mass of zinc Determination of 0.899 2) Moles of zinc 0.01361 mol 3.) Moles of hydrogen 00? ← I was told to calculate this number from mole of zinc. 350m So does that mean it will be 0.01361 mol too? 4 Volume of water collected (mL) 5) VL of water collected (Liters) 0.350 L 6) Temp of water collected (°C) 7) Temp of water collected (°K) 8) Atmospheric pressure (mm) 9) Vapor pressure of water (mm) 10) Corrected pressure of hydrogen 20% 29°C 764.0mm Hg (mm) 17.5mm 11) Corrected pressure of hydrogen (atm) 12) Experimentally calculated value of 19 13. Literature value of R 14) % Error 15) Suggest reasons for the % error (#14)arrow_forwardNo wedge or dashes. Do proper structure. Provide steps and explanation.arrow_forward10 Question (1 point) Draw curved arrow notation to indicate the proton transfer between NaOH and CH3CO₂H. 2nd attempt :0- H See Periodic Table See Hint Draw the products of the proton transfer reaction. Don't add a + sign between the products.arrow_forward
- Nonearrow_forward4. Experimental Procedure. a. How many (total) data plots are to be completed for this experiment? Account for each. b. What information is to be extracted from each data plot?arrow_forwardProvide the IUPAC name of the following molecule. Don't forget to include the proper stereochemistry where appropriate.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning