
Concept explainers
Expand the list of orbitais considered in Figures 5.2 and 5.3 by using all three p orbitals of atom A and all five d orbitals of atom B. Which of these have the necessary match ofsymmetry for bonding and antibondingorbitals? These combinations are rarely seen in simplemolecules but can be important in
Interpretation: The list of orbitals should be expanded using all three p-orbitals of atom A and all five d-orbitals of atom B and the orbitals should be selected which has necessary match of symmetry for bonding and antibonding orbitals.
Concept Introduction: Three conditions are considered for overlapping which leads to bonding.
- The orbital’s symmetry must be such that regions with the same sign of ψ overlap.
- The energies of atomic orbital must be comparable.
- In order to provide good overlap, the distance between the atoms should be short enough but not so short as the repulsion of other electrons or nuclei occur.
Answer to Problem 5.1P
Bonding interactions -
P orbitals and
Explanation of Solution
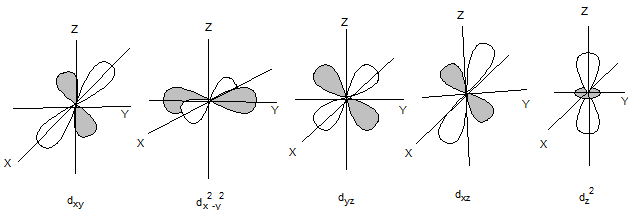
The shapes of p orbitals are as follows:
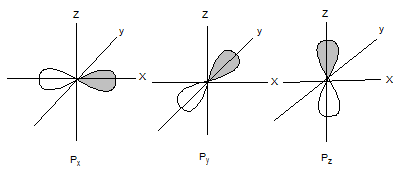
The shapes of d orbitals are as follows:
There are three possible bonding interactions. They are,
P orbitals and
Want to see more full solutions like this?
Chapter 5 Solutions
EBK INORGANIC CHEMISTRY
Additional Science Textbook Solutions
Campbell Biology in Focus (2nd Edition)
Organic Chemistry
Applications and Investigations in Earth Science (9th Edition)
Human Biology: Concepts and Current Issues (8th Edition)
Campbell Biology (11th Edition)
- NH2 1. CH3–MgCl 2. H3O+ ? As the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C - C bond as its major product: If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new C - C bond, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. This reaction will not make a product with a new C - C bond. Х ☐: Carrow_forwardPredict the major products of this organic reaction. If there will be no major products, check the box under the drawing area instead. No reaction. : + Х è OH K Cr O 2 27 2 4' 2 Click and drag to start drawing a structure.arrow_forwardLaminar compounds are characterized by havinga) a high value of the internal surface of the solid.b) a high adsorption potential.arrow_forward
- Intercalation compounds have their sheetsa) negatively charged.b) positively charged.arrow_forwardIndicate whether the following two statements are correct or not:- Polythiazine, formed by N and S, does not conduct electricity- Carbon can have a specific surface area of 3000 m2/garrow_forwardIndicate whether the following two statements are correct or not:- The S8 heterocycle is the origin of a family of compounds- Most of the elements that give rise to stable heterocycles belong to group d.arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning


