
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
9th Edition
ISBN: 9780136781776
Author: Wade
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5.10C, Problem 5.19P
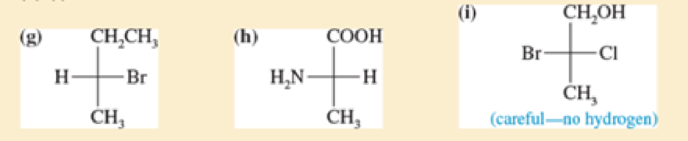
For each Fischer projection, label each asymmetric carbon atom as (R) or (S) (a)-(f) the structures in Problem5-18
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
When natural light falls perpendicularly on a material A, it has a reflectivity of 0.813%. Indicate the value of the refractive index.
In piezoelectricity and piezoelectric ceramics, one of the following options is false:(A). Piezoelectricity allows an electrical signal to be transformed into a mechanical one.(B). PbZrO3 is a well-known piezoelectric ceramic.(C). Piezoelectricity and ferroelectricity in general have no relationship.(D). One of the applications of piezoelectricity is sonar.
(30 MARKS) Give the major product(s
) formed including relevant
stereochemistry or the complete
reaction conditions for the following
reactions. More than one step may be
required for each reaction arrow, in
which case the steps must be
numbered 1), 2) etc. (2 marks each
box) h)
i)
h)
OH
i)
HO
H3PO4, heat
2
Br
Chapter 5 Solutions
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
Ch. 5.2 - Determine whether the following objects are chiral...Ch. 5.2A - Prob. 5.2PCh. 5.2B - Prob. 5.3PCh. 5.2B - Prob. 5.4PCh. 5.2C - Prob. 5.5PCh. 5.3 - Prob. 5.6PCh. 5.3 - Prob. 5.7PCh. 5.4D - Prob. 5.8PCh. 5.4D - Prob. 5.9PCh. 5.4D - Prob. 5.10P
Ch. 5.5 - Prob. 5.11PCh. 5.7 - When optically pure (R)-2-bromobutane is heated...Ch. 5.7 - Prob. 5.13PCh. 5.8 - Prob. 5.14PCh. 5.9B - Draw three-dimensional representations of the...Ch. 5.10A - For each sot of examples, make a model of the...Ch. 5.10A - Draw a Fischer projection for each compound....Ch. 5.10B - Prob. 5.18PCh. 5.10C - For each Fischer projection, label each asymmetric...Ch. 5.11C - Prob. 5.20PCh. 5.13 - Prob. 5.21PCh. 5.13 - Prob. 5.22PCh. 5.15 - Prob. 5.23PCh. 5.16A - Prob. 5.24PCh. 5 - The following four structures are naturally...Ch. 5 - For each structure, 1. star () any asymmetric...Ch. 5 - Prob. 5.27SPCh. 5 - Prob. 5.28SPCh. 5 - Prob. 5.29SPCh. 5 - Prob. 5.30SPCh. 5 - Prob. 5.31SPCh. 5 - Prob. 5.32SPCh. 5 - Prob. 5.33SPCh. 5 - Prob. 5.34SPCh. 5 - For each structure, 1. draw all the stereoisomers....Ch. 5 - Prob. 5.36SPCh. 5 - Prob. 5.37SPCh. 5 - 3,4-Dimethylpent-1-ene has the formula...Ch. 5 - A graduate student was studying enzymatic...Ch. 5 - Prob. 5.40SPCh. 5 - Prob. 5.41SP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
What process causes the Mediterranean intermediate Water MIW to become more dense than water in the adjacent At...
Applications and Investigations in Earth Science (9th Edition)
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Define histology.
Fundamentals of Anatomy & Physiology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonearrow_forwardIndicate which option is false(A). Resistivity has a residual component and a thermal component.(B). In some materials resistivity increases with T and in others it decreases.(C). In insulating materials, resistivity is very low.arrow_forwardIn ceramic materials, in relation to polymorphism, the same substance crystallizes differently when external conditions vary. Is this correct?arrow_forward
- Indicate the type of bond that is considered to be a hydrogen bond.(A). Permanent dipole-dipole interaction between polar molecules.(B). Mixed ionic-covalent bond.(C). Principal interatomic bond(D). Van del Waals forces.arrow_forwardRetro aldol: NaOH H₂O H NaOH & d H₂O Harrow_forwardDraw the product of the reaction shown below. Ignore inorganic byproducts. H conc. HBr Drawing Qarrow_forward
- Calculate the atomic packing factor of diamond knowing that the number of Si atoms per cm3 is 2.66·1022 and that the atomic radii of silicon and oxygen are, respectively, 0.038 and 0.117 nm.arrow_forwardA pdf file of your hand drawn, stepwise mechanisms for the reactions. For each reaction in the assignment, you must write each mechanism three times (there are 10 reactions, so 30 mechanisms). (A) do the work on a tablet and save as a pdf., it is expected to write each mechanism out and NOT copy and paste the mechanism after writing it just once. Everything should be drawn out stepwise and every bond that is formed and broken in the process of the reaction, and is expected to see all relevant lone pair electrons and curved arrows. Aldol: NaOH HO H Δ NaOH Δarrow_forwardNonearrow_forward
- Draw structures corresponding to the following names and give IUPAC names for the following compounds: (8 Point) a) b) c) CH3 CH2CH3 CH3CHCH2CH2CH CH3 C=C H3C H H2C=C=CHCH3 d) CI e) (3E,5Z)-2,6-Dimethyl-1,3,5,7-octatetraene f) (Z)-4-bromo-3-methyl-3-penten-1-yne g) cis-1-Bromo-2-ethylcyclopentane h) (5R)-4,4,5-trichloro-3,3-dimethyldecanearrow_forwardNonearrow_forwardReview: Design a total total synthesis synthesis of the following compound using methyloxacyclopropane and any other necessary reagents.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License