
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
9th Edition
ISBN: 9780136781776
Author: Wade
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 5.26SP
For each structure,
- 1. star (*) any asymmetric carbon atoms.
- 2. label each asymmetric carbon as (R) or (S).
- 3. draw any internal mirror planes of symmetry.
- 4. label the structure as chiral or achiral.
- 5. label any meso structures.
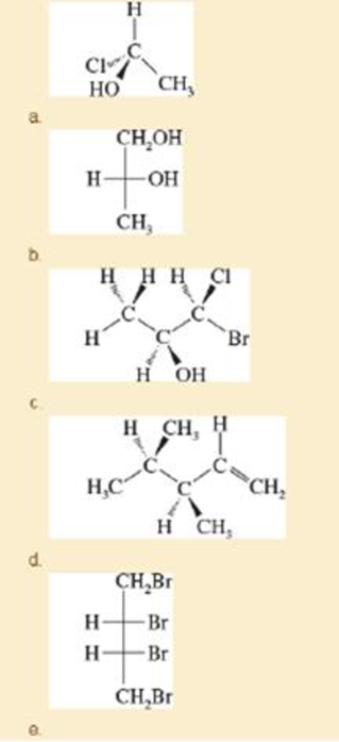
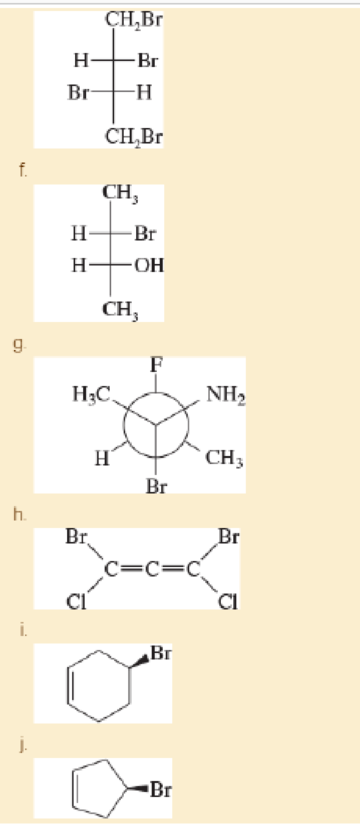
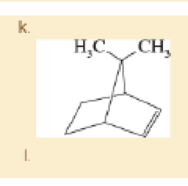
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Convert the following structures into a chair representation. Then conduct a chair flip.
Cl
a.
b.
C\....
о
Aktiv Learning App
Cengage Digital Learning
Part of Speech Table for Assign x
o
Mail-Karen Ento-Outlook
* +
app.aktiv.com
Your Aktiv Learning trial expires on 02/06/25 at 01:15 PM
Curved arrows are used to illustrate the flow of electrons. Using
the provided starting and product structures, draw the curved
electron-pushing arrows for the following reaction or
mechanistic step(s).
Be sure to account for all bond-breaking and bond-making
steps.
Problem 17 of 30
Drawing Arrows
heat
4
O
M
B
D
5x
H
H
Und Settings
H
Done
:0:
H
Jar
Convert the following chairs into ring representations:
a.
Brz
b.
Chapter 5 Solutions
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
Ch. 5.2 - Determine whether the following objects are chiral...Ch. 5.2A - Prob. 5.2PCh. 5.2B - Prob. 5.3PCh. 5.2B - Prob. 5.4PCh. 5.2C - Prob. 5.5PCh. 5.3 - Prob. 5.6PCh. 5.3 - Prob. 5.7PCh. 5.4D - Prob. 5.8PCh. 5.4D - Prob. 5.9PCh. 5.4D - Prob. 5.10P
Ch. 5.5 - Prob. 5.11PCh. 5.7 - When optically pure (R)-2-bromobutane is heated...Ch. 5.7 - Prob. 5.13PCh. 5.8 - Prob. 5.14PCh. 5.9B - Draw three-dimensional representations of the...Ch. 5.10A - For each sot of examples, make a model of the...Ch. 5.10A - Draw a Fischer projection for each compound....Ch. 5.10B - Prob. 5.18PCh. 5.10C - For each Fischer projection, label each asymmetric...Ch. 5.11C - Prob. 5.20PCh. 5.13 - Prob. 5.21PCh. 5.13 - Prob. 5.22PCh. 5.15 - Prob. 5.23PCh. 5.16A - Prob. 5.24PCh. 5 - The following four structures are naturally...Ch. 5 - For each structure, 1. star () any asymmetric...Ch. 5 - Prob. 5.27SPCh. 5 - Prob. 5.28SPCh. 5 - Prob. 5.29SPCh. 5 - Prob. 5.30SPCh. 5 - Prob. 5.31SPCh. 5 - Prob. 5.32SPCh. 5 - Prob. 5.33SPCh. 5 - Prob. 5.34SPCh. 5 - For each structure, 1. draw all the stereoisomers....Ch. 5 - Prob. 5.36SPCh. 5 - Prob. 5.37SPCh. 5 - 3,4-Dimethylpent-1-ene has the formula...Ch. 5 - A graduate student was studying enzymatic...Ch. 5 - Prob. 5.40SPCh. 5 - Prob. 5.41SP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Describe the evolution of mammals, tracing their synapsid lineage from early amniote ancestors to true mammals....
Loose Leaf For Integrated Principles Of Zoology
2. Which of the following is the best example of the use of a referent? _
a. A red bicycle
b. Big as a dump tru...
Physical Science
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
Some people consider Pasteur or Koch to be the Father of Microbiology, rather than Leeuwenhoek. Why might they ...
Microbiology with Diseases by Body System (5th Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Drawing Arrows 1 I I 1 heat 1 51 MO + Drag To Und Settings Done 0 0 Jan 31 3:5arrow_forwardDon't used hand raitingarrow_forwardGramicidin A can adopt more than one structure; NMR spectroscopy has revealed an “end-to-end” dimer form, and x-ray crystallography has revealed an “anti-parallel double- helical” form. Briefly outline and describe an experimentalapproach/strategy to investigate WHICH configuration (“end-to-end dimer” vs “anti-paralleldouble helical”) gramicidin adopts in an actual lipid bilayer.arrow_forward
- Don't used hand raitingarrow_forwardCHEM2323 Problem 2-24 Tt O e: ל Predict the product(s) of the following acid/base reactions. Draw curved arrows to show the formation and breaking of bonds. If the bonds needed are not drawn out, you should redraw them. + BF3 (a) (b) HI + (c) OH -BF Problem 2-25 Use curved arrows and a proton (H+) to draw the protonated form of the following Lewis bases. Before starting, add all missing lone pairs. (a) (b) :0: (c) N 1 CHEM2323 PS CH02 Name:arrow_forwardCHEM2323 Problem 2-26 Tt O PS CH02 Name: Use the curved-arrow formalism to show how the electrons flow in the resonance form on the left to give the one on the right. (Draw all lone pairs first) (a) NH2 NH2 + (b) Problem 2-27 Double bonds can also act like Lewis bases, sharing their electrons with Lewis acids. Use curved arrows to show how each of the following double bonds will react with H-Cl and draw the resulting carbocation. (a) H2C=CH2 (b) (c) Problem 2-28 Identify the most electronegative element in each of the following molecules: (a) CH2FCI F Problem 2-29 (b) FCH2CH2CH2Br (c) HOCH2CH2NH2 (d) CH3OCH2Li F 0 0 Use the electronegativity table in Figure 2.3 to predict which bond in the following pairs is more polar and indicate the direction of bond polarity for each compound. (a) H3C-Cl or Cl-CI (b) H3C-H or H-CI (c) HO-CH3 or (CH3)3Si-CH3 (d) H3C-Li or Li-OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License