
Concept explainers
(a)
Interpretation:
The structure of compound X with the molecular formula
Concept introduction:
The cis – trans (E/Z) isomer can be predicted with respect to the double bond. The isomers can be predicted with respect to the substituents present in carbon – carbon double bond. If the substituents are present on the same side, it is called cis (or) Z isomer. If the substituents are present on opposite sides, it is called Trans (or) E isomer.
Example:
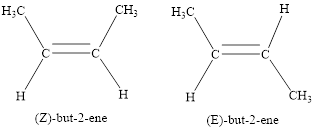
The stereoisomerism is the arrangement of atoms in molecules whose connectivity remains the same but their arrangement in different in each isomer.
The two molecules are described as stereoisomers if they are made of the same atoms connected in the same sequence, but the atoms are positions differently in space.
Chiral centre: A chiral centre is defined as the tetrahedral carbon atom in an organic molecule that is connected to four non-identical groups/substituents. It is sometimes known as a stereo genic centre.
The carbon atom bonded to four unlike groups is called an asymmetric carbon atom and is also known as chiral carbon.
(b)
Interpretation:
The structure of compound Y with the molecular formula
Concept introduction:
The cis – Trans (E/Z) isomer can be predicted with respect to the double bond. The isomers can be predicted with respect to the substituents present in carbon – carbon double bond. If the substituents are present on the same side, it is called cis (or) Z isomer. If the substituents are present on opposite sides, it is called trans (or) E isomer.
Example:

The stereoisomerism is the arrangement of atoms in molecules whose connectivity remains the same but their arrangement in different in each isomer.
The two molecules are described as stereoisomers if they are made of the same atoms connected in the same sequence, but the atoms are positions differently in space.
Chiral centre: A chiral centre is defined as the tetrahedral carbon atom in an organic molecule that is connected to four non-identical groups/substituents. It is sometimes known as a stereo genic centre.
The carbon atom bonded to four unlike groups is called an asymmetric carbon atom and is also known as chiral carbon.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic Chemistry
- 5. Write the formation reaction of the following complex compounds from the following reactants: 6. AgNO₃ + K₂CrO₂ + NH₄OH → 7. HgNO₃ + excess KI → 8. Al(NO₃)₃ + excess NaOH →arrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. CO₂C2H5 + CH3-NH-NH,arrow_forwardDraw the major product of this reaction N-(cyclohex-1-en-1-yl)-1-(pyrrolidino) reacts with CH2=CHCHO, heat, H3O+arrow_forward
- Draw the starting material that would be needed to make this product through an intramolecular Dieckmann reactionarrow_forwardDraw the major product of this reaction. Nitropropane reacts + pent-3-en-2-one reacts with NaOCH2CH3, CH3CHOHarrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. OC2H5 + CoHs-NH-NH,arrow_forward
- Explain how substitutions at the 5-position of barbituric acid increase the compound's lipophilicity.arrow_forwardExplain how substitutions at the 5-position of phenobarbital increase the compound's lipophilicity.arrow_forwardName an interesting derivative of barbituric acid, describing its structure.arrow_forward
- Briefly describe the synthesis mechanism of barbituric acid from the condensation of urea with a β-diketone.arrow_forwardGiven the hydrazones indicated, draw the structures of the enamines that can be formed. Indicate the most stable enamine (explain). C6H5 C6H5 H C6H5 Harrow_forward4. Propose a Synthesis for the molecule below. You may use any starting materials containing 6 carbons or less (reagents that aren't incorporated into the final molecule such as PhзP do not count towards this total, and the starting material can have whatever non-carbon functional groups you want), and any of the reactions you have learned so far in organic chemistry I, II, and III. Your final answer should show each step separately, with intermediates and conditions clearly drawn.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





