
Concept explainers
Interpretation:
Each
Concept introduction:
Cis-trans isomerism: In a compound with a Carbon-Carbon double bond, geometric isomerism is possible. The plane which is perpendicular to the pi-orbitals is considered the reference. There are a total of four substituents, two each at either of the carbons indulged in the double bond.
When two of the heaviest substituents are oriented on the same side of the reference plane, the geometric isomer is termed a ‘cis’-isomer.
When two of the heaviest substituents are oriented on the opposite sides of the reference plane, the geometric isomer is termed a ‘trans’-isomer.
Geometric isomers: Two atoms with the same side location of the double bond are called cis isomers and two atoms with opposite side locations of the double bond are called Trans isomers.
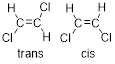
In a cyclic compound: The spatial orientation of the di substituents cyclic compound must be viewed as a three-dimensional conformation. Generally, a solid wedge bond indicates it is above the plane of the ring and a broken wedge bond indicates it is below the plane.
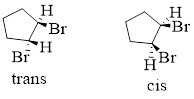
The stereoisomerism is the arrangement of atoms in molecules whose connectivity remains the same but their arrangement in different in each isomer.
The two molecules are described as stereoisomers if they are made of the same atoms connected in the same sequence, but the atoms are positions differently in space.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic Chemistry, 3e WileyPLUS Registration Card + Loose-leaf Print Companion
- at 32.0 °C? What is the osmotic pressure (in atm) of a 1.46 M aqueous solution of urea [(NH2), CO] at 3 Round your answer to 3 significant digits.arrow_forwardReagan is doing an atomic absorption experiment that requires a set of zinc standards in the 0.4-1.6 ppm range. A 1000 ppm Zn solution was prepared by dissolving the necessary amount of solid Zn(NO3)2 in water. The standards can be prepared by diluting the 1000 ppm Zn solution. Table 1 shows one possible set of serial dilutions (stepwise dilution of a solution) that Reagan could perform to make the necessary standards. Solution A was prepared by diluting 5.00 ml of the 1000 ppm Zn standard to 50.00 ml. Solutions C-E are called "calibration standards" because they will be used to calibrate the atomic absorption spectrometer. a. Compare the solution concentrations expressed as ppm Zn and ppm Zn(NO3)2. Compare the concentrations expressed as M Zn and M Zn(NO3)2 - Which units allow easy conversion between chemical species (e.g. Zn and Zn(NO3)2)? - Which units express concentrations in numbers with easily expressed magnitudes? - Suppose you have an analyte for which you don't know the molar…arrow_forwardNonearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





