
Concept explainers
(a)
The initial and the final temperature of the piston cylinder device.
(a)
Explanation of Solution
Given:
The total mass of the mixture
The initial pressure
The mass of the water
The mass of the water vapor
The final pressure of the system
The final volume of the system
Calculation:
Calculate the total initial volume of piston cylinder device.
Here, the specific volume of saturated liquid is
Calculate the total volume of the piston cylinder device at final state.
Calculate the specific volume of the piston cylinder device at final state.
Here, the mass of the saturated liquid vapour mixture of water is contained in a piston cylinder device is
Calculation:
Write the formula of interpolation method of two variables.
Here, the variables denote by x and y is saturated pressure and saturated temperature.
For initial temperature of the piston cylinder device.
Show the temperature at pressure of 150 kPa, 160 kPa, and 175 kPa as in Table (1).
|
Pressure, kPa |
Temperature, C |
| 150 kPa | 111.35 |
| 160 kPa | |
| 175 kPa | 116.04 |
Substitute the value of x and y from Table (1) in Equation (IV) to calculate the value of initial temperature
Thus, the initial temperature of the piston cylinder device is
For specific volume of saturated liquid of the piston cylinder device.
Show the specific volume of saturated liquid at pressure of 150 kPa, 160 kPa, and 175 kPa as in Table (2).
|
Pressure, kPa |
Specific volume of saturated liquid, |
| 150 kPa | 0.001053 |
| 160 kPa | |
| 175 kPa | 0.001057 |
Substitute the value of x and y from Table (2) in Equation (IV) to calculate the value of specific volume of saturated liquid
For specific volume of saturated vapour of the piston cylinder device.
Show the specific volume of saturated vapour at pressure of 150 kPa, 160 kPa, and 175 kPa as in Table (3).
|
Pressure, kPa |
Specific volume of saturated vapour, |
| 150 kPa | 1.1594 |
| 160 kPa | |
| 175 kPa | 1.0037 |
Substitute the value of x and y from Table (3) in Equation (IV) to calculate the value of specific volume of saturated vapour
Substitute
Substitute
Substitute
The unit conversion of pressure from kPa to MPa.
For temperature of the piston cylinder device at final state.
Show the temperature at specific volume of the piston cylinder device at final state at
|
specific volume of the piston cylinder device at final state, |
Temperature, |
| 600 | |
| 700 |
Substitute the value of x and y from Table (4) in Equation (IV) to calculate the value of temperature of the piston cylinder device at final state
Thus, the final temperature of the piston cylinder device is
(b)
The mass of liquid water when the piston first starts moving.
(b)
Explanation of Solution
Since,
Calculate the specific volume of the piston cylinder device at this state.
Therefore, the value of specific volume of the piston cylinder device at this state is greater than
Thus, the mass of liquid water when the piston first starts moving is
(c)
The work done during the process state 2 and 3.
(c)
Explanation of Solution
Calculate the work done in constant pressure process.
Thus, the work done during the process state 2 and 3 is
Show the P-v diagram of this process.
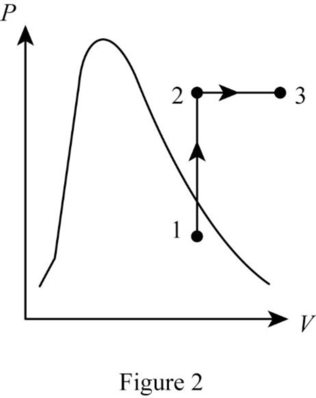
Want to see more full solutions like this?
Chapter 5 Solutions
Fundamentals Of Thermal-fluid Sciences In Si Units
- Hello I was going over the solution for this probem and I'm a bit confused on the last part. Can you please explain to me 1^4 was used for the Co of the tubular cross section? Thank you!arrow_forwardBlood (HD = 0.45 in large diameter tubes) is forced through hollow fiber tubes that are 20 µm in diameter.Equating the volumetric flowrate expressions from (1) assuming marginal zone theory and (2) using an apparentviscosity for the blood, estimate the marginal zone thickness at this diameter. The viscosity of plasma is 1.2 cParrow_forwardQ2: Find the shear load on bolt A for the connection shown in Figure 2. Dimensions are in mm Fig. 2 24 0-0 0-0 A 180kN (10 Markarrow_forward
- determine the direction and magnitude of angular velocity ω3 of link CD in the four-bar linkage using the relative velocity graphical methodarrow_forwardFour-bar linkage mechanism, AB=40mm, BC=60mm, CD=70mm, AD=80mm, =60°, w1=10rad/s. Determine the direction and magnitude of w3 using relative motion graphical method. A B 2 3 77777 477777arrow_forwardFour-bar linkage mechanism, AB=40mm, BC=60mm, CD=70mm, AD=80mm, =60°, w1=10rad/s. Determine the direction and magnitude of w3 using relative motion graphical method. A B 2 3 77777 477777arrow_forward
- The evaporator of a vapor compression refrigeration cycle utilizing R-123 as the refrigerant isbeing used to chill water. The evaporator is a shell and tube heat exchanger with the water flowingthrough the tubes. The water enters the heat exchanger at a temperature of 54°F. The approachtemperature difference of the evaporator is 3°R. The evaporating pressure of the refrigeration cycleis 4.8 psia and the condensing pressure is 75 psia. The refrigerant is flowing through the cycle witha flow rate of 18,000 lbm/hr. The R-123 leaves the evaporator as a saturated vapor and leaves thecondenser as a saturated liquid. Determine the following:a. The outlet temperature of the chilled waterb. The volumetric flow rate of the chilled water (gpm)c. The UA product of the evaporator (Btu/h-°F)d. The heat transfer rate between the refrigerant and the water (tons)arrow_forward(Read image) (Answer given)arrow_forwardProblem (17): water flowing in an open channel of a rectangular cross-section with width (b) transitions from a mild slope to a steep slope (i.e., from subcritical to supercritical flow) with normal water depths of (y₁) and (y2), respectively. Given the values of y₁ [m], y₂ [m], and b [m], calculate the discharge in the channel (Q) in [Lit/s]. Givens: y1 = 4.112 m y2 = 0.387 m b = 0.942 m Answers: ( 1 ) 1880.186 lit/s ( 2 ) 4042.945 lit/s ( 3 ) 2553.11 lit/s ( 4 ) 3130.448 lit/sarrow_forward
- Problem (14): A pump is being used to lift water from an underground tank through a pipe of diameter (d) at discharge (Q). The total head loss until the pump entrance can be calculated as (h₁ = K[V²/2g]), h where (V) is the flow velocity in the pipe. The elevation difference between the pump and tank surface is (h). Given the values of h [cm], d [cm], and K [-], calculate the maximum discharge Q [Lit/s] beyond which cavitation would take place at the pump entrance. Assume Turbulent flow conditions. Givens: h = 120.31 cm d = 14.455 cm K = 8.976 Q Answers: (1) 94.917 lit/s (2) 49.048 lit/s ( 3 ) 80.722 lit/s 68.588 lit/s 4arrow_forwardProblem (13): A pump is being used to lift water from the bottom tank to the top tank in a galvanized iron pipe at a discharge (Q). The length and diameter of the pipe section from the bottom tank to the pump are (L₁) and (d₁), respectively. The length and diameter of the pipe section from the pump to the top tank are (L2) and (d2), respectively. Given the values of Q [L/s], L₁ [m], d₁ [m], L₂ [m], d₂ [m], calculate total head loss due to friction (i.e., major loss) in the pipe (hmajor-loss) in [cm]. Givens: L₁,d₁ Pump L₂,d2 오 0.533 lit/s L1 = 6920.729 m d1 = 1.065 m L2 = 70.946 m d2 0.072 m Answers: (1) 3.069 cm (2) 3.914 cm ( 3 ) 2.519 cm ( 4 ) 1.855 cm TABLE 8.1 Equivalent Roughness for New Pipes Pipe Riveted steel Concrete Wood stave Cast iron Galvanized iron Equivalent Roughness, & Feet Millimeters 0.003-0.03 0.9-9.0 0.001-0.01 0.3-3.0 0.0006-0.003 0.18-0.9 0.00085 0.26 0.0005 0.15 0.045 0.000005 0.0015 0.0 (smooth) 0.0 (smooth) Commercial steel or wrought iron 0.00015 Drawn…arrow_forwardThe flow rate is 12.275 Liters/s and the diameter is 6.266 cm.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





