
Concept explainers
(a)
The initial and the final temperature of the piston cylinder device.
(a)
Explanation of Solution
Given:
The total mass of the mixture
The initial pressure
The mass of the water
The mass of the water vapor
The final pressure of the system
The final volume of the system
Calculation:
Calculate the total initial volume of piston cylinder device.
Here, the specific volume of saturated liquid is
Calculate the total volume of the piston cylinder device at final state.
Calculate the specific volume of the piston cylinder device at final state.
Here, the mass of the saturated liquid vapour mixture of water is contained in a piston cylinder device is
Calculation:
Write the formula of interpolation method of two variables.
Here, the variables denote by x and y is saturated pressure and saturated temperature.
For initial temperature of the piston cylinder device.
Show the temperature at pressure of 150 kPa, 160 kPa, and 175 kPa as in Table (1).
|
Pressure, kPa |
Temperature, C |
| 150 kPa | 111.35 |
| 160 kPa | |
| 175 kPa | 116.04 |
Substitute the value of x and y from Table (1) in Equation (IV) to calculate the value of initial temperature
Thus, the initial temperature of the piston cylinder device is
For specific volume of saturated liquid of the piston cylinder device.
Show the specific volume of saturated liquid at pressure of 150 kPa, 160 kPa, and 175 kPa as in Table (2).
|
Pressure, kPa |
Specific volume of saturated liquid, |
| 150 kPa | 0.001053 |
| 160 kPa | |
| 175 kPa | 0.001057 |
Substitute the value of x and y from Table (2) in Equation (IV) to calculate the value of specific volume of saturated liquid
For specific volume of saturated vapour of the piston cylinder device.
Show the specific volume of saturated vapour at pressure of 150 kPa, 160 kPa, and 175 kPa as in Table (3).
|
Pressure, kPa |
Specific volume of saturated vapour, |
| 150 kPa | 1.1594 |
| 160 kPa | |
| 175 kPa | 1.0037 |
Substitute the value of x and y from Table (3) in Equation (IV) to calculate the value of specific volume of saturated vapour
Substitute
Substitute
Substitute
The unit conversion of pressure from kPa to MPa.
For temperature of the piston cylinder device at final state.
Show the temperature at specific volume of the piston cylinder device at final state at
|
specific volume of the piston cylinder device at final state, |
Temperature, |
| 600 | |
| 700 |
Substitute the value of x and y from Table (4) in Equation (IV) to calculate the value of temperature of the piston cylinder device at final state
Thus, the final temperature of the piston cylinder device is
(b)
The mass of liquid water when the piston first starts moving.
(b)
Explanation of Solution
Since,
Calculate the specific volume of the piston cylinder device at this state.
Therefore, the value of specific volume of the piston cylinder device at this state is greater than
Thus, the mass of liquid water when the piston first starts moving is
(c)
The work done during the process state 2 and 3.
(c)
Explanation of Solution
Calculate the work done in constant pressure process.
Thus, the work done during the process state 2 and 3 is
Show the P-v diagram of this process.
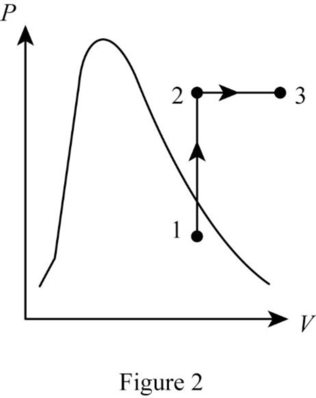
Want to see more full solutions like this?
Chapter 5 Solutions
Fundamentals of Thermal-Fluid Sciences
- **Problem 8-45.** The man has a mass of 60 kg and the crate has a mass of 100 kg. If the coefficient of static friction between his shoes and the ground is \( \mu_s = 0.4 \) and between the crate and the ground is \( \mu_c = 0.3 \), determine if the man is able to move the crate using the rope-and-pulley system shown. **Diagram Explanation:** The diagram illustrates a scenario where a man is attempting to pull a crate using a rope-and-pulley system. The setup is as follows: - **Crate (C):** Positioned on the ground with a rope attached. - **Rope:** Connects the crate to a pulley system and extends to the man. - **Pulley on Tree:** The rope runs over a pulley mounted on a tree which redirects the rope. - **Angles:** - The rope between the crate and tree forms a \(30^\circ\) angle with the horizontal. - The rope between the tree and the man makes a \(45^\circ\) angle with the horizontal. - **Man (A):** Pulling on the rope with the intention of moving the crate. This arrangement tests the…arrow_forwardplease solve this problems follow what the question are asking to do please show me step by steparrow_forwardplease first write the line action find the forces and them solve the problem step by steparrow_forward
- please solve this problem what the problem are asking to solve please explain step by step and give me the correct answerarrow_forwardplease help me to solve this problem step by steparrow_forwardplease help me to solve this problem and determine the stress for each point i like to be explained step by step with the correct answerarrow_forward
- please solve this problem for me the best way that you can explained to solve please show me the step how to solvearrow_forwardplese solbe this problem and give the correct answer solve step by step find the forces and line actionarrow_forwardplease help me to solve this problems first write the line of action and them find the forces {fx=0: fy=0: mz=0: and them draw the shear and bending moment diagram. please explain step by steparrow_forward
- please solve this problem step by step like human and give correct answer step by steparrow_forwardPROBLEM 11: Determine the force, P, that must be exerted on the handles of the bolt cutter. (A) 7.5 N (B) 30.0 N (C) 52.5 N (D) 300 N (E) 325 N .B X 3 cm E 40 cm cm F = 1000 N 10 cm 3 cm boltarrow_forwardUsing the moment-area theorems, determine a) the rotation at A, b) the deflection at L/2, c) the deflection at L/4. (Hint: Use symmetry for Part a (θA= - θB, or θC=0), Use the rotation at A for Parts b and c. Note that all deformations in the scope of our topics are small deformation and for small θ, sinθ=θ).arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





