
Concept explainers
The following figure shows three 1.00-L bulbs connected by valves. Each bulb contains argon gas with amounts proportional to the number of circles pictorially represented in the chamber. All three bulbs are maintained at the same temperature. Unless stated otherwise, assume that the valves connecting the bulbs are closed and seal the gases in their respective chambers. Assume also that the volume between each bulb is negligible.
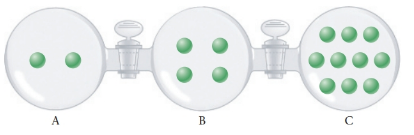
(a) Which bulb has the highest pressure?
(b) If the pressure in bulb A is 0.500 atm, what is the pressure in bulb C?
(c) If the pressure in bulb A is 0.500 atm, what is the total pressure?
(d) If the pressure in bulb A is 0.500 arm, and the valve between bulbs A and B is opened, redraw the figure shown above to accurately represent the gas atoms in all three bulbs. What is
(e) Follow the instructions of part (d) but now open only the valve between bulbs B and C.
(a)
Interpretation:
The bulb with the highest pressure needs to be identified based on the given description.
Concept introduction:
The ideal gas equation is a thermodynamic equation of state which relates the pressure (P), volume (V), number of moles (n) and temperature (T) of an ideal gas through the following expression:
where R is the universal gas constant = 0.0821 L.atm/mol-K
Answer to Problem 86QAP
Bulb C has the highest pressure.
Explanation of Solution
Given Information:
Volume (V) of bulb A = B = C = 1.0 L
Temperature (T) of bulb A = B = C
Number of moles (n) of Ar gas in A = 2
Number of moles (n) of Ar gas in B = 4
Number of moles (n) of Ar gas in C = 10
Calculation:
Based on equation (1), the pressure (P) in each of the bulbs can de deduced by substituting the values of n in bulbs A, B and C and volume, V = 1.0 L
Now, pressure (P) is directly proportional to the number of moles (n). Therefore, under constant temperature, bulb C will have the highest pressure.
(b)
Interpretation:
The pressure in bulb C needs to be deduced if the pressure in bulb A is 0.500 atm.
Concept introduction:
The ideal gas equation is a thermodynamic equation of state which relates the pressure (P), volume (V), number of moles (n) and temperature (T) of an ideal gas through the following expression:
where R is the universal gas constant = 0.0821 L.atm/mol-K
Answer to Problem 86QAP
Pressure in Bulb C is 2.5 atm
Explanation of Solution
Given Information:
Volume (V) of bulb A = B = C = 1.0 L
Temperature (T) of bulb A = B = C
Pressure (P) in bulb A = 0.500 atm
Number of moles (n) of Ar gas in A = 2
Number of moles (n) of Ar gas in C = 10
Calculation:
Based on equation (1), the pressure (P) in bulbs A and C can be deduced by substituting the given values of n, V and P under constant T
(c)
Interpretation:
The total pressure needs to be deduced if the pressure in bulb A is 0.500 atm.
Concept introduction:
The ideal gas equation is a thermodynamic equation of state which relates the pressure (P), volume (V), number of moles (n) and temperature (T) of an ideal gas through the following expression:
where R is the universal gas constant = 0.0821 L.atm/mol-K
As per Dalton’s law, the total pressure exerted by a gas mixture is equal to the sum of the partial pressure of the individual gases.
Answer to Problem 86QAP
Total pressure = 4.00 atm
Explanation of Solution
Given Information:
Volume (V) of bulb A = B = C = 1.0 L
Temperature (T) of bulb A = B = C
Pressure (P) in bulb A = 0.500 atm
Number of moles (n) of Ar gas in A = 2
Number of moles (n) of Ar gas in B = 4
Number of moles (n) of Ar gas in C = 10
Calculation:
Based on equation (1), the pressure (P) in bulbs A and B can be deduced by substituting the given values of n, V and P under constant T
(d)
Interpretation:
The total pressure needs to be deduced after the valve between A and B is opened.
Concept introduction:
The ideal gas equation is a thermodynamic equation of state which relates the pressure (P), volume (V), number of moles (n) and temperature (T) of an ideal gas through the following expression:
where R is the universal gas constant = 0.0821 L.atm/mol-K
As per Dalton’s law, the total pressure exerted by a gas mixture is equal to the sum of the partial pressure of the individual gases.
Answer to Problem 86QAP
Total pressure after the valve between A and B is opened in 8.50 atm
Explanation of Solution
Given Information:
Volume (V) of bulb A = B = C = 1.0 L
Temperature (T) of bulb A = B = C
Pressure (P) in bulb A = 0.500 atm
Number of moles (n) of Ar gas in A = 2
Number of moles (n) of Ar gas in B = 4
Number of moles (n) of Ar gas in C = 10
Calculation:
When the valve between A and B is opened the Ar gas will diffuse from the region of high pressure i.e. bulb B to A until equilibrium is established.
Now the total number of moles (atoms) of Ar = 2 + 4 = 6. The final pressure in each bulb will be due to 6 moles (atoms) of Ar
Step 1: Calculate the final pressure in bulb A after mixing:
The initial pressure in bulb A = Pi = 0.500 atm
Initial moles of Ar gas in A = ni = 2
Final moles in A = nf = 6
The final pressure in bulb A = Pf
Under constant V and T, the ratio of the initial and final pressures would be:
Step 2: Calculate the final pressure in bulb B after mixing:
The initial pressure in bulb B = Pi = 1.50 atm
Initial moles of Ar gas in A = ni = 2
Final moles in A = nf = 6
The final pressure in bulb A = Pf
Under constant V and T, the ratio of the initial and final pressures would be:
Step 3: Calculate the total pressure after mixing:
The total pressure after the valve between A and B is opened is higher than that when the valve is closed.
(d)
Interpretation:
The total pressure needs to be deduced after the valve between B and C is opened.
Concept introduction:
The ideal gas equation is a thermodynamic equation of state which relates the pressure (P), volume (V), number of moles (n) and temperature (T) of an ideal gas through the following expression:
where R is the universal gas constant = 0.0821 L.atm/mol-K
As per Dalton’s law, the total pressure exerted by a gas mixture is equal to the sum of the partial pressure of the individual gases.
Answer to Problem 86QAP
Total pressure after the valve between B and C is opened in 9.25 atm
Explanation of Solution
Given Information:
Volume (V) of bulb A = B = C = 1.0 L
Temperature (T) of bulb A = B = C
Pressure (P) in bulb A = 0.500 atm
Number of moles (n) of Ar gas in A = 2
Number of moles (n) of Ar gas in B = 4
Number of moles (n) of Ar gas in C = 10
Calculation:
When the valve between B and C is opened the Ar gas will diffuse from the region of high pressure i.e. bulb C to B until equilibrium is established.
Now the total number of moles (atoms) of Ar = 4 + 10 = 14. The final pressure in each bulb will be due to 14 moles (atoms) of Ar
Step 1: Calculate the final pressure in bulb B after mixing:
The initial pressure in bulb B = Pi = 1.50 atm
Initial moles of Ar gas in B = ni = 4
Final moles in A = nf = 10
The final pressure in bulb B = Pf
Under constant V and T, the ratio of the initial and final pressures would be:
Step 2: Calculate the final pressure in bulb C after mixing:
The initial pressure in bulb C = Pi = 2.50 atm
Initial moles of Ar gas in C = ni = 10
Final moles in C = nf = 14
The final pressure in bulb A = Pf
Under constant V and T, the ratio of the initial and final pressures would be:
Step 3: Calculate the total pressure after mixing:
The total pressure after the valve between B and C is opened is higher than that when the valve is closed.
Want to see more full solutions like this?
Chapter 5 Solutions
OWLV2 FOR MASTERTON/HURLEY'S CHEMISTRY:
- SH 0 iq noitzouDarrow_forwardNonearrow_forward+ HCl →? Draw the molecule on the canvas by choosing buttons from the Tools (for bonas), Atoms and Advanced Template toolbars. The single bond is active by default. + M C + H± 2D EXP. CONT. K ? L 1 H₁₂C [1] A HCN O S CH3 CH 3 CI Br HC H₂ CH CH CH3 - P Farrow_forward
- SHarrow_forwardSH 0arrow_forward2. Please consider the two all 'cis' isomers of trimethylcyclohexane drawn below. Draw the two chair conformers of each stereoisomer below (1 and 2) and calculate their torsional interaction energies in order to identify the lower energy conformer for each stereoisomer. Based on your calculations, state which of the two stereoisomers 1 and 2 is less stable and which is more stable. [1,3-diaxial CH3 CH3 = 3.7kcal/mol; 1,3-diaxial CH3 H = 0.88kcal/mol; cis-1,2 (axial:equatorial) CH3 CH3 = 0.88kcal/mol; trans-1,2-diequatorial CH3 CH3 = 0.88kcal/mol) all-cis-1,2,3- 1 all-cis-1,2,4- 2arrow_forward
- Nonearrow_forwardWhat is the mechanism by which the 1,4 product is created? Please draw it by hand with arrows and stuff.arrow_forwardWhat is the relationship between A and B? H3C A Br Cl H3C B Br relationship (check all that apply) O same molecule O enantiomer O diastereomer structural isomer O stereoisomer isomer O need more information to decide O same molecule ☐ enantiomer Br Br Br CH3 Br CI CH3 O diastereomer ☐ structural isomer ☐ stereoisomer isomer O need more information to decide O same molecule O enantiomer Odiastereomer structural isomer O stereoisomer ☐ isomer O need more information to decidearrow_forward
- b. Please complete the zig-zag conformation of the compound (3R,4S)-3,4-dichloro-2,5-dimethylhexane by writing the respective atoms in the boxes. 4arrow_forwardc. Serricornin, the female-produced sex pheromone of the cigarette beetle, has the following structure. OH What is the maximum number of possible stereoisomers? Is this structure a meso compound? d. Please consider the natural product alkaloids shown below. Are these two structures enantiomers, diastereomers or conformers? H HO H H HN HO HN R R с R=H cinchonidine R=ET cinchonine Harrow_forwardNail polish remover containing acetone was spilled in a room 5.23 m × 3.28 m × 2.76 m. Measurements indicated that 2,250 mg of acetone evaporated. Calculate the acetone concentration in micrograms per cubic meter.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





