
Concept explainers
Interpretation:
The possible stereoisomers for the given compound has to be drawn.
Concept introduction:
Cis-trans isomerism: In a compound with a Carbon-Carbon double bond, geometric isomerism is possible. The plane which is perpendicular to the pi-orbitals is considered the reference. There are a total of four substituents, two each at either of the carbons indulged in the double bond.
When two of the heaviest substituents are oriented on the same side of the reference plane, the geometric isomer is termed a ‘cis’-isomer.
When two of the heaviest substituents are oriented on the opposite sides of the reference plane, the geometric isomer is termed a ‘trans’-isomer.
Geometric isomers: Two atoms with the same side location of the double bond are called cis isomers and two atoms with opposite side locations of the double bond are called Trans isomers.
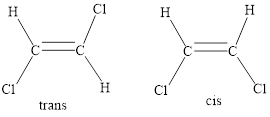
The stereoisomerism is the arrangement of atoms in molecules whose connectivity remains the same but their arrangement in different in each isomer.
The two molecules are described as stereoisomers if they are made of the same atoms connected in the same sequence, but the atoms are positions differently in space.
Chiral centre: A chiral center is defined as the tetrahedral carbon atom in an organic molecule that is connected to four non-identical groups/substituents. It is sometimes known as a stereo genic center.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
ORGANIC CHEMISTRY, WITH SOL. MAN/ STUDY
- Nonearrow_forwardPart II. Given below are the 'H-NMR spectrum at 300 MHz in CDC13 and mass spectrum using electron ionization of compound Brian. The FTIR of the said compound showed a strong peak at 1710 cm"). Determine the following: (a) molecular Formula and Degree of unsaturation of compound Brian (b) Basing on the given H-NMR spectrum tabulate the following (i) chemical shifts (ii) integration, ciii) multiplicity and (iv) interferences made for each signal (c) Draw the structure of compound Brian. ) ΕΙ 43 41 27 71 114 (M+) Hmmm 20 30 40 50 60 70 80 90 100 110 120 1H NMR spectrum 300 MHz in CDCl3 2.0 alle 1.0arrow_forwardThe iron-iron carbide phase diagram. In the diagram, the letter L indicates that it is a liquid. Indicate what its components are. Temperature (°C) 1600 10 Composition (at% C) 15 25 1538°C -1493°C 8 1400 1200 1394°C y+L L 2500 1147°C y. Austenite 2.14 4.30 2000 1000 912°C y + Fe3C 800ㅏ 0.76 0.022 600 a, Ferrite a + Fe3C 400 0 (Fe) Composition (wt% C) 727°C 1500 Cementite (Fe3C) 1000 6 6.70 Temperature (°F)arrow_forward
- Part V. Choose which isomer would give the 1H-NMR spectrum below. Justify your reasoning by assigning important signals to the Corresponding protons of the correct molecule. A D on of of of H H 88 2 90 7.8 7.6 7.4 80 5 6 [ppm] 7.2 6.8 6.6 6.4 ō [ppm]arrow_forwardShow work with explanation. don't give Ai generated solutionarrow_forwardQ7. a. Draw the line-bond structure of the major product for the following reaction, if a reaction occurs, assume monohalogenation. b. Calculate the product ratios using the following information (hint: use the number of hydrogens in each category present to calculate the ratios). Chlorination: 1° Reactivity=1 2° Reactivity=4 Heat + Cl2 3° Reactivity=5arrow_forward
- Please correct answer and don't use hand rating and don't use Ai solutionarrow_forwardQ10: Alkane halogenation a. Give the name and structures of the five isomeric hexanes. Page 4 of 5 Chem 0310 Organic Chemistry 1 Recitations b. For each isomer, give all the free radical monochlorination and monobromination products that are structurally isomeric.arrow_forwardQ9. The insecticide DDT (in the box below) is useful in controlling mosquito populations and has low toxicity to humans, but is dangerous to birds and fish. Hoping to alleviate the dangers, little Johnny Whizbang, an aspiring chemist, proposes a new version of DDT ("Bromo-DDT") and shows his synthesis to his boss. Will Johnny Whizbang's synthesis work? Or will he be fired? Assume there is an excess of bromine and polybrominated products can be separated. Explain why. CH3 Br2, light CBR3 ok-ok Br Br Br Br CI "Bromo-DDT" CCl 3 DDT (dichlorodiphenyltrichloroethane) CIarrow_forward
- Differentiate the terms Monotectic, Eutectic, Eutectoid, Peritectic, Peritectoid.arrow_forwardQ5. Predict the organic product(s) for the following transformations. If no reaction will take place (or the reaction is not synthetically useful), write "N.R.". Determine what type of transition state is present for each reaction (think Hammond Postulate). I Br₂ CH3 F2, light CH3 Heat CH3 F₂ Heat Br2, light 12, light CH3 Cl2, lightarrow_forwarda. For the following indicated bonds, rank them in order of decreasing AH° for homolytic cleavage. Based on your answer, which bond would be most likely to break homolytically? (a) (c) H3C CH3 .CH3 CH3 CH3 (b) Page 1 of 5 Chem 0310 Organic Chemistry 1 Recitations b. Draw all the possible radical products for 2-methylbutane, and determine which bond is most likely to be broken.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





