
A ?owchart of a methanol synthesis process is shown below.
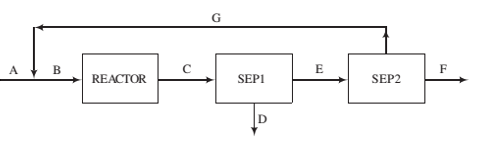
The following speci?cations apply to the labeled streams and process units:
A. Fresh feed—a mixture of CO, H2, N2, and CO2
B. Feed to the reactor—30.0 mole% CO, 63.0% H2, 2.0% N2, and 5.0% CO2.
Reactor. Two reactions occur and proceed to equilibrium at 200°C and 4925 kPa absolute:
C. Reactor effluent—contains all feed and product species at the reactor temperature and pressure. Species partial pressures satisfy the two given equations.
Sep1. Condenses all methanol and water in reactor ef?uent.
D. Liquid methanol and water, (These species will be separated by distillation in a unit not shown.)
E. Gas containing N2and unreacted CO, H2, and CO2.
Sep2. Multiple-unit separation process.
F. All of the nitrogen and some of the hydrogen in Stream E.
G. Recycle stream—CO, CO2, and 10% of the hydrogen fed to Sep2.
(a) Taking 100 kmol/h of Stream B as a basis of calculation, calculate the molar ?ow rates (kmol/h) and molar compositions of the remaining six labeled streams.
(b) The process is to be used to provide 237 kmol/h of methanol. Scale up the ?owchart of Part (a) to calculate the required fresh feed rate (SCMH), the ?ow rate of the reactor ef?uent (SCMH), and the actual volumetric ?ow rate of the reactor ef?uent (m3/h), assuming ideal-gas behavior.
(c) Use the rule of thumb for a diatomic gas given in Equation 5.2-3 to test the ideal-gas assumption at the reactor outlet. If the assumption is invalid, which of the values calculated in Part (b) are in error?
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Elementary Principles of Chemical Processes
Additional Engineering Textbook Solutions
Elementary Surveying: An Introduction To Geomatics (15th Edition)
INTERNATIONAL EDITION---Engineering Mechanics: Statics, 14th edition (SI unit)
Starting Out with C++ from Control Structures to Objects (9th Edition)
Starting Out with Python (4th Edition)
Introduction To Programming Using Visual Basic (11th Edition)
Problem Solving with C++ (10th Edition)
- The power out of an adiabatic steam turbine is 5 MW and the steam enters turbine at 2 MPa and velocity of 50 m/s, specific enthalpy (h) of 3248 kJ/kg. The elevation of the inlet is 10 m higher than at the datum. The vapor mixture exits at 15 kPa and a velocity of 180 m/s, specific enthalpy (h) of 2361.01 kJ/kg. The elevation of the exit is 6 m higher than at the datum. Let g = 9.81 m/s². Assuming the ideal gas model and R = 0.462 KJ/(kg.K). The steam specific heat ratio is 1.283. Calculate:arrow_forwardThe power out of an adiabatic steam turbine is 5 MW and the steam enters turbine at 2 MPa and velocity of 50 m/s, specific enthalpy (h) of 3248 kJ/kg. The elevation of the inlet is 10 m higher than at the datum. The vapor mixture exits at 15 kPa and a velocity of 180 m/s, specific enthalpy (h) of 2361.01 kJ/kg. The elevation of the exit is 6 m higher than at the datum. Let g = 9.81 m/s². Assuming the ideal gas model and R = 0.462 KJ/(kg.K). The steam specific heat ratio is 1.283. Calculate:arrow_forwardO Consider a 0.8 m high and 0.5 m wide window with thickness of 8 mm and thermal conductivity of k = 0.78 W/m °C. For dry day, the temperature of outdoor is -10 °C and the inner room temperature is 20°C. Take the heat transfer coefficient on the inner and outer surface of the window to be h₁ = 10 W/m² °C and h₂ = 40 W/m² °C which includes the effects of insulation. Determine:arrow_forward
- 4. Show that the fraction, F, of the energy released from a supercritical chain reaction that originates in the final m generations of the chain is given approximately by F= 1 km provided the total number of generations is large.arrow_forwardPLEASE SOLVE STEP BY STEP WITHOUT ARTIFICIAL INTELLIGENCE OR CHATGPT I don't understand why you use chatgpt, if I wanted to I would do it myself, I need to learn from you, not from being a d amn robot. SOLVE BY HAND STEP BY STEP A solution containing 7.5% sulfuric acid by weight at 70 °F is concentrated to 45% by weight by evaporating water. The concentrated solution and the water vapor exit the evaporator at 170 °F and 1 atm. Calculate the rate at which heat must be transferred to the evaporator to process 1500 lbm/hr of the feed solution to the evaporator. It is recommended to use the enthalpy-concentration diagram for sulfuric acid from Chapter 8 of Felder's book or an enthalpy-concentration diagram for sulfuric acid found in another unit operations book or chemical engineering manual such as Perry's.arrow_forwardPLEASE SOLVE STEP BY STEP WITHOUT ARTIFICIAL INTELLIGENCE OR CHATGPT I don't understand why you use chatgpt, if I wanted to I would do it myself, I need to learn from you, not from being a d amn robot. SOLVE BY HAND STEP BY STEP Suppose that the system designed in problem 33 of the Thermodynamics II problem set from UAM-Azcapotzalco is relocated to another area near the sea, specifically, Ciudad del Carmen, Campeche. Recalculate the compressor-cooler system for the new environmental conditions. Make the considerations you deem logical in redesigning your system. Indicate the references or sources where you obtained your data.arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





