
Bread is typically made by ?rst dissolving preserved yeast (a microscopic biological organism that consumes sugars and emits CO2 as a waste product) in water, then adding other ingredients, including ?our, sugar, fat (usually butter or shortening), and salt. After the ingredients are combined, the dough is "kneaded," or mixed to promote the formation of a protein network from two proteins (gliadin and glutenin)15 present in wheat ?our. This network is what strengthens the dough and allows it to stretch elastically without breaking. The dough is then allowed to rise in a process called "proo?ng," in which the yeast consumes sugar and releases CO2, which in?ates air pockets in the dough that are subsequently ?lled with air. Finally the dough is baked; the gas pockets expand due to the temperature rise and evaporation of water, the starches from the ?our are dehydrated (dried), and the yeast dies.
A good French bread has an open, porous structure. The pores must be stabilized by the protein network until the bread is dried suf?ciently to hold its shape. The bread collapses if the protein network fails prematurely.
(a) Rouille et al.16 investigated the in?uence of ingredients and mixing conditions on the quality of frozen French bread dough. Each loaf was initially formed roughly as a cylinder with a mass of 150 g (including essentially no CO2), a diameter of 2.0 cm. and a length of 25.0cm. Determine the speci?c volume of a bread dough proofed for two hours at 28°C from which 1.20 cm3 gas/min per 100 g dough evolves as bubbles within the dough. State your assumptions.
(b) During proo?ng, the increases in volume of a series of control loaves were monitored along with the mass of CO2 evolved. Rupture of the protein network during proo?ng can be detected when the volume of the dough no longer increases at the same rate as the production of CO2 from the yeast. Data from one of these experiments are shown in the table below. Plot the speci?c volumes of CO2 (per 100g dough) and dough as a function of time. If the preferred proo?ng time is such that the dough achieves 70% of its total volume before collapse, specify the proper proo?ng time for this formula.
(c) The referenced study found that the parameter with the most signi?cant in?uence on dough quality was mixing time, with an extended mixing time producing a stronger protein network. Why might extended mixing times not be desirable in commercial production of bread?
(d) Suggest causes for the following undesirable bread-baking outcomes: (i) a ?at, dense loaf; (ii) an overly large loaf.
(e) Suggest why the period during which the dough rises is called "proo?ng." Remember that yeast is a biological organism.
| t(min) | 0 | 20 | 40 | 60 | 80 | 100 | 120 | 140 | 160 | 180 | 200 | 220 | 240 |
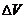 cm3 dough) cm3 dough) |
0 | 0 | 20 | 60 | 80 | 115 | 155 | 198 | 247 | 305 | 322 | 334 | 336 |
| Gas evolved (g CO2) | 0.0 | 37.2 | 63.2 | 68.8 | 126.3 | 192.7 | 234.8 | 315.8 | 385.4 | 515.0 | 578.1 | 657.5 | 745.0 |
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Elementary Principles of Chemical Processes
Additional Engineering Textbook Solutions
Elementary Surveying: An Introduction To Geomatics (15th Edition)
Java How to Program, Early Objects (11th Edition) (Deitel: How to Program)
Electric Circuits. (11th Edition)
Computer Science: An Overview (13th Edition) (What's New in Computer Science)
Modern Database Management
Management Information Systems: Managing The Digital Firm (16th Edition)
- The power out of an adiabatic steam turbine is 5 MW and the steam enters turbine at 2 MPa and velocity of 50 m/s, specific enthalpy (h) of 3248 kJ/kg. The elevation of the inlet is 10 m higher than at the datum. The vapor mixture exits at 15 kPa and a velocity of 180 m/s, specific enthalpy (h) of 2361.01 kJ/kg. The elevation of the exit is 6 m higher than at the datum. Let g = 9.81 m/s². Assuming the ideal gas model and R = 0.462 KJ/(kg.K). The steam specific heat ratio is 1.283. Calculate:arrow_forwardstep by step pleasearrow_forwardstep by step pleasearrow_forward
- step by steparrow_forwardThe power out of an adiabatic steam turbine is 5 MW and the steam enters turbine at 2 MPa and velocity of 50 m/s, specific enthalpy (h) of 3248 kJ/kg. The elevation of the inlet is 10 m higher than at the datum. The vapor mixture exits at 15 kPa and a velocity of 180 m/s, specific enthalpy (h) of 2361.01 kJ/kg. The elevation of the exit is 6 m higher than at the datum. Let g = 9.81 m/s². Assuming the ideal gas model and R = 0.462 KJ/(kg.K). The steam specific heat ratio is 1.283. Calculate:arrow_forwardThe power out of an adiabatic steam turbine is 5 MW and the steam enters turbine at 2 MPa and velocity of 50 m/s, specific enthalpy (h) of 3248 kJ/kg. The elevation of the inlet is 10 m higher than at the datum. The vapor mixture exits at 15 kPa and a velocity of 180 m/s, specific enthalpy (h) of 2361.01 kJ/kg. The elevation of the exit is 6 m higher than at the datum. Let g = 9.81 m/s². Assuming the ideal gas model and R = 0.462 KJ/(kg.K). The steam specific heat ratio is 1.283. Calculate:arrow_forward
- O Consider a 0.8 m high and 0.5 m wide window with thickness of 8 mm and thermal conductivity of k = 0.78 W/m °C. For dry day, the temperature of outdoor is -10 °C and the inner room temperature is 20°C. Take the heat transfer coefficient on the inner and outer surface of the window to be h₁ = 10 W/m² °C and h₂ = 40 W/m² °C which includes the effects of insulation. Determine:arrow_forwardCalculate the mass flow rate of the steam. Determine Cp and C₁ of steam.arrow_forwardstep by step pleasearrow_forward
- step by steparrow_forward4. Show that the fraction, F, of the energy released from a supercritical chain reaction that originates in the final m generations of the chain is given approximately by F= 1 km provided the total number of generations is large.arrow_forwardPLEASE SOLVE STEP BY STEP WITHOUT ARTIFICIAL INTELLIGENCE OR CHATGPT I don't understand why you use chatgpt, if I wanted to I would do it myself, I need to learn from you, not from being a d amn robot. SOLVE BY HAND STEP BY STEP A solution containing 7.5% sulfuric acid by weight at 70 °F is concentrated to 45% by weight by evaporating water. The concentrated solution and the water vapor exit the evaporator at 170 °F and 1 atm. Calculate the rate at which heat must be transferred to the evaporator to process 1500 lbm/hr of the feed solution to the evaporator. It is recommended to use the enthalpy-concentration diagram for sulfuric acid from Chapter 8 of Felder's book or an enthalpy-concentration diagram for sulfuric acid found in another unit operations book or chemical engineering manual such as Perry's.arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





