
Concept explainers
(a)
Interpretation:
Fischer projection of the given molecule is to be drawn.
Concept introduction:
A molecule containing a chain of carbon atoms is frequently represented in its zigzag conformation. If it contains multiple asymmetric centers, it is more convenient to draw its Fisher projection. Fischer projections are generally drawn with the longest carbon chain vertical. The two groups attached to each carbon except the first and last are shown on horizontal bonds. The carbon atoms in the middle of the chain are represented by the intersections of vertical and horizontal lines. The vertical lines represent bonds that are oriented away from the viewer while the horizontal bonds are oriented toward the viewer. The most oxidized group must be present at the top of the vertical line.
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the same side of the plane and are shown by wedge bonds, then those groups will be on the opposite sides of the vertical chain in the Fischer projection.
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the opposite sides of the plane and are shown by a wedge and a dash bond, then, in the Fischer projection, those groups will be on the same side of the vertical chain.
In the zig-zag conformation, if two groups on alternate carbon atoms are on opposite sides of the plane and are shown by a wedge and a dash bond, then, in the Fischer projection, those groups will be on the opposite sides of the vertical chain.
Answer to Problem 5.52P
The Fischer projection of the given molecule is
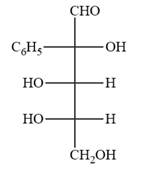
Explanation of Solution
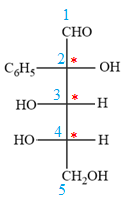
The given molecule is
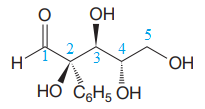
The given molecule consists of a five carbon chain, each of which is numbered. C2, C3, and C4 are the asymmetric carbon atoms. We begin to draw the Fischer projection with the framework shown below:
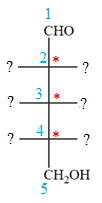
The asymmetric centers are denoted by asterisks. Two of the bonds on each asymmetric center have been left with question marks because two substituents must still be added to each so that the stereochemical configuration at those carbon atoms in the Fischer projection matches with what was given in the dash-wedge notation.
Notice that in the zig-zag conformation, the OH groups on C2 and C3 carbon atoms lie on the same side of the plane and are shown by a wedge bond. Thus, these two groups must lie on opposite sides in the Fischer projection, as shown below:
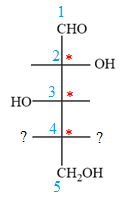
The OH groups on C3 and C4 carbon atoms lie on the opposite side of the plane and are shown by a wedge and a dash bond. Thus, these two groups must lie on the same side in the Fischer projection, as shown below:
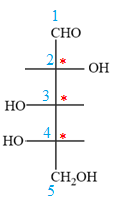
The OH groups on C2 and C4 carbon atoms lie on the opposite side of the plane and are shown by a wedge and a dash bond. Thus, these two groups must lie on the opposite side in the Fischer projection.
Thus, the OH groups on C3 and C4 carbon atoms must lie on the same side of the vertical chain, and OH group on C2 must lie on the opposite side. Then fill in the remaining groups on C2, C3, and C4 carbon atoms to complete the structure as below:
Thus, the structure above is the correct conversion from a given zig-zag conformation to a Fischer projection.
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the opposite sides of the plane and are shown by a wedge and dash bond, then those groups will be on the same side of the vertical chain in the Fischer projection. In the zig-zag conformation, if two groups on adjacent carbon atoms are on opposite sides of the plane and are shown by a wedge and dash bond, then those groups will be on the same side of the vertical chain in the Fischer projection.
Interpretation:
(b)
Fischer projection of the given molecule is to be drawn.
Concept introduction:
A molecule containing a chain of carbon atoms is frequently represented in its zigzag conformation. If it contains multiple asymmetric centers, it is more convenient to draw its Fisher projection. Fischer projections are generally drawn with the longest carbon chain vertical. The two groups attached to each carbon except the first and last are shown on horizontal bonds. The carbon atoms in the middle of the chain are represented by the intersections of vertical and horizontal lines. The vertical lines represent bonds that are oriented away from the viewer while the horizontal bonds are oriented toward the viewer. The most oxidized group must be present at the top of the vertical line.
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the same side of the plane and are shown by wedge bonds, then those groups will be on the opposite sides of the vertical chain in the Fischer projection.
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the opposite sides of the plane and are shown by a wedge and a dash bond, then, in the Fischer projection, those groups will be on the same side of the vertical chain.
In the zig-zag conformation, if two groups on alternate carbon atoms are on opposite sides of the plane and are shown by a wedge and a dash bond, then, in the Fischer projection, those groups will be on the opposite sides of the vertical chain.
Answer to Problem 5.52P
The Fischer projection of the given molecule is
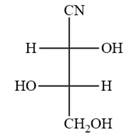
Explanation of Solution
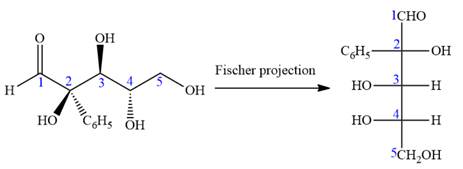
The given molecule is
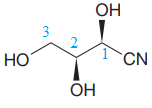
The given molecule consists of a three carbon chain, each of which is numbered. C1 and C2 carbon atoms are the asymmetric carbon atoms. We begin to draw the Fischer projection with the framework shown below:
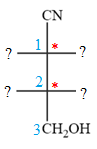
The asymmetric centers are denoted by asterisks. Two of the bonds on each asymmetric center have been left with question marks because two substituents must still be added to each so that the stereochemical configuration at those carbon atoms in the Fischer projection matches with what was given in the dash-wedge notation.
Notice that in the zig-zag conformation, the OH groups on C1 and C2 carbon atoms lie on the same side of the plane and are shown by a wedge bond. Thus, these two groups must lie on opposite side in the Fischer projection, as shown below:
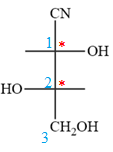
Then fill in the remaining groups on C1 and C2 carbon atoms to complete the structure as below:
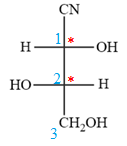
Thus, the structure above is the correct conversion from a given zig-zag conformation to a Fischer projection.
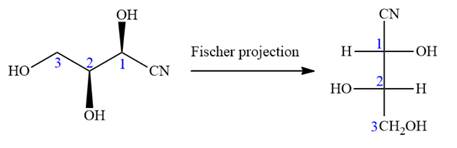
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the opposite sides of the plane and are shown by a wedge and dash bond, then those groups will be on the same side of the vertical chain in the Fischer projection. In the zig-zag conformation, if two groups on adjacent carbon atoms are on opposite sides of the plane and are shown by a wedge and dash bond, then those groups will be on the same side of the vertical chain in the Fischer projection.
Interpretation:
(c)
Fischer projection of the given molecule is to be drawn.
Concept introduction:
A molecule containing a chain of carbon atoms is frequently represented in its zigzag conformation. If it contains multiple asymmetric centers, it is more convenient to draw its Fisher projection. Fischer projections are generally drawn with the longest carbon chain vertical. The two groups attached to each carbon except the first and last are shown on horizontal bonds. The carbon atoms in the middle of the chain are represented by the intersections of vertical and horizontal lines. The vertical lines represent bonds that are oriented away from the viewer while the horizontal bonds are oriented toward the viewer. The most oxidized group must be present at the top of the vertical line.
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the same side of the plane and are shown by wedge bonds, then those groups will be on the opposite sides of the vertical chain in the Fischer projection.
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the opposite sides of the plane and are shown by a wedge and a dash bond, then, in the Fischer projection, those groups will be on the same side of the vertical chain.
In the zig-zag conformation, if two groups on alternate carbon atoms are on opposite sides of the plane and are shown by a wedge and a dash bond, then, in the Fischer projection, those groups will be on the opposite sides of the vertical chain.
Answer to Problem 5.52P
The Fischer projection of the given molecule is
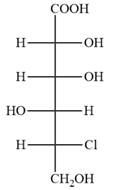
Explanation of Solution
The given molecule is
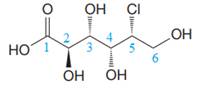
The given molecule consists of a six carbon chain, each of which is numbered. C2, C3, C4, and C5 carbon atoms are the asymmetric carbon atoms. We begin to draw the Fischer projection with the framework shown below:

The asymmetric centers are denoted by asterisks. Two of the bonds on each asymmetric center have been left with question marks because two substituents must still be added to each so that the stereochemical configuration at those carbon atoms in the Fischer projection matches with what was given in the dash-wedge notation.
Notice that in the zig-zag conformation, the OH groups on C2 and C3 carbon atoms lie on the opposite side of the plane and are shown by a wedge and a dash bond. Thus, these two groups must lie on same side in the Fischer projection, as shown below:
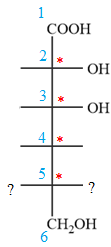
Notice that in the zig-zag conformation, the OH groups on C3 and C4 carbon atoms lie on the same side of the plane and are shown by dash bonds. Thus, these two groups must lie on the opposite sides of the vertical chain in the Fischer projection, as shown below:
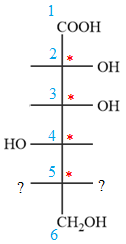
In the zig-zag conformation, the OH group and chlorine atom on C4 and C5 carbon atoms lie on the same side of the plane and are shown by dash bonds. Thus, these two groups must lie on the opposite sides of the vertical chain in the Fischer projection, as shown below:
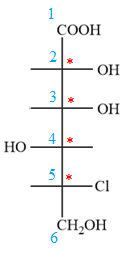
Then place the remaining groups on C1 and C2 carbon atoms to complete the structure as below:
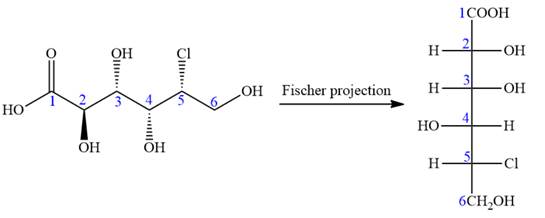
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the opposite sides of the plane and are shown by a wedge and dash bond, then those groups will be on the same side of the vertical chain in the Fischer projection. In the zig-zag conformation, if two groups on adjacent carbon atoms are on opposite sides of the plane and are shown by a wedge and dash bond, then those groups will be on the same side of the vertical chain in the Fischer projection.
Want to see more full solutions like this?
Chapter 5 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- Nonearrow_forwardNonearrow_forwardn Feb 3 A T + 4. (2 pts) Draw the structure of the major component of the Limonene isolated. Explain how you confirmed the structure. 5. (2 pts) Draw the fragment corresponding to the base peak in the Mass spectrum of Limonene. 6. (1 pts) Predict the 1H NMR spectral data of R-Limonene. Proton NMR: 5.3 pon multiplet (H Ringarrow_forward
- Part VI. Ca H 10 O is the molecular formula of compound Tom and gives the in the table below. Give a possible structure for compound Tom. 13C Signals summarized C1 C2 C3 C4 C5 C6 C7 13C shift (ppm) 23.5 27.0 33.0 35.8 127 162 205 DEPT-90 + DEPT-135 + +arrow_forward2. Using the following data to calculate the value of AvapH o of water at 298K. AvapH o of water at 373K is 40.7 kJ/mol; molar heat capacity of liquid water at constant pressure is 75.2J mol-1 K-1 and molar heat capacity of water vapor at constant pressure is 33.6 J mol-1 K-1.arrow_forwardPart VII. Below are the 'HNMR 13 3 C-NMR, COSY 2D- NMR, and HSQC 20-NMR (Similar with HETCOR but axes are reversed) spectra of an organic compound with molecular formula C6H13 O. Assign chemical shift values to the H and c atoms of the compound. Find the structure. Show complete solutions. Predicted 1H NMR Spectrum ли 4.7 4.6 4.5 4.4 4.3 4.2 4.1 4.0 3.9 3.8 3.7 3.6 3.5 3.4 3.3 3.2 3.1 3.0 2.9 2.8 2.7 2.6 2.5 2.4 2.3 2.2 2.1 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1 1.0 0.9 0.8 f1 (ppm)arrow_forward
- 3. Draw the expanded structural formula, the condensed structural formula, and the skeletal structural formula for 2-pentene. expanded structure: Condensed structure: Skeletal formula: 4. Draw the expanded structural formula, the condensed structural formula, and the skeletal structural formula for 2-methyl-3-heptene. expanded structure: Condensed structure: Skeletal formula: following structurearrow_forwardPart IV. Propose a plausible Structure w/ the following descriptions: a) A 5-carbon hydrocarbon w/ a single peak in its proton decoupled the DEPT-135 Spectrum shows a negative peak C-NMR spectrum where b) what cyclohexane dione isomer gives the largest no. Of 13C NMR signals? c) C5H120 (5-carbon alcohol) w/ most deshielded carbon absent in any of its DEPT Spectivaarrow_forward13C NMR is good for: a) determining the molecular weight of the compound b) identifying certain functional groups. c) determining the carbon skeleton, for example methyl vs ethyl vs propyl groups d) determining how many different kinds of carbon are in the moleculearrow_forward
- 6 D 2. (1 pt) Limonene can be isolated by performing steam distillation of orange peel. Could you have performed this experiment using hexane instead of water? Explain. 3. (2 pts) Using GCMS results, analyze and discuss the purity of the Limonene obtained from the steam distillation of orange peel.arrow_forwardPart III. Arrange the following carbons (in blue) in order of increasing chemical shift. HO B NH 2 A CIarrow_forward6. Choose the compound that will produce the spectrum below and assign the signals as carbonyl, aryl, or alkyl. 100 ō (ppm) 50 0 7. 200 150 Assign all of the protons on the spectrum below. 8. A B 4 E C 3 ō (ppm) 2 1 0 Choose the compound that will produce the spectrum below and assign the signals to the corresponding protons. OH 6 OH 3 2 1 0 4 ō (ppm)arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
