
Concept explainers
To determine: The diagram of the new nucleus when the given isotope emits a positron
Answer to Problem 5.51UTC
Solution:
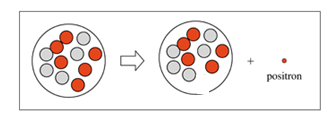
The new nucleus when the given isotopes emit a positron is as follows:

Explanation of Solution
Isotopes:
The atoms of an element with different mass number or
Therefore it in not necessary that all atoms of a given element contain the same number of neutrons in their nuclei . For example hydrogen has three isotopes
Positron particle (
To balance the nuclear reaction as follows:
- The sum of the subscript means the
atomic numbers must be equal on the both side of the equation. - The sum of the superscript means the mass numbers must be equal on the both side of the equation.
Calculation:
When an atom of X-11 emits a positron then Y-11 is formed. The balanced nuclear reaction is as follows:
Therefore; the new nucleus when the given isotopes emit a positron; has same in mass number and 1 unit’s decreases in atomic number as follows:

Want to see more full solutions like this?
Chapter 5 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry Plus Mastering Chemistry with Pearson eText -- Access Card Package (13th Edition)
- How will you prepare the following buffers? 2.5 L of 1.5M buffer, pH = 10.5 from NH4Cl and NH3arrow_forwardCH₂O and 22 NMR Solvent: CDCl3 IR Solvent: neat 4000 3000 2000 1500 1000 15 [ اند 6,5 9.8 3.0 7.0 6.0 5.0 4.8 3.0 2.0 1.0 9.8 200 100arrow_forwardprotons. Calculate the mass (in grams) of H3AsO4 (MW=141.9416) needed to produce 3.125 x 1026arrow_forward
- Using what we have learned in CHEM 2310 and up through class on 1/31, propose a series of reaction steps to achieve the transformation below. Be sure to show all reagents and intermediates for full credit. You do not need to draw mechanism arrows, but you do need to include charges where appropriate. If you do not put your group name, you will get half credit at most. ? Brarrow_forwardDraw a mechanism for the formation of 2-bromovanillin using bromonium ion as the reactive electrophile.arrow_forwardNonearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





