
GENERAL ORGANIC+BIO...(LL)-W/MOD.ACCESS
3rd Edition
ISBN: 9780134466699
Author: FROST
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 5.38PP
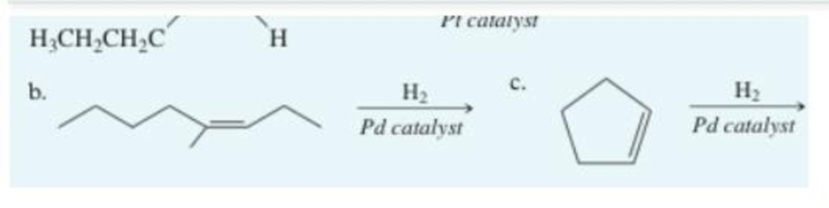
Write the main product of hydration for the
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In one electrode: Pt, H2(1 atm) | H+(a=1), the interchange current density at 298K is 0.79 mA·cm-2. If the voltage difference of the interface is +5 mV. What will be the correct intensity at pH = 2?. Maximum transfer voltage and beta = 0.5.
In a Pt electrode, H2(1 atm) | H+(a=1), the interchange current density of an electrode is 0.79 mA cm-2. ¿Qué corriente flow across the electrode of área 5 cm2 when the difference in potential of the interface is +5 mV?.
If the current voltage is n = 0.14 V, indicate which of the 2 voltage
formulas of the ley of Tafel must be applied
i
a
a) == exp (1-B).
xp[(1 - ß³):
Fn
Fn
a
b) == exp B
RT
RT
Chapter 5 Solutions
GENERAL ORGANIC+BIO...(LL)-W/MOD.ACCESS
Ch. 5 - When vinegar (CH3COOH) and baking soda (NaHCO3)...Ch. 5 - In your own words, define free energy change, G....Ch. 5 - Classify the following as exothermic or...Ch. 5 - Prob. 5.4PPCh. 5 - Prob. 5.5PPCh. 5 - Classify the following as spontaneous or...Ch. 5 - Prob. 5.7PPCh. 5 - Prob. 5.8PPCh. 5 - Prob. 5.9PPCh. 5 - Prob. 5.10PP
Ch. 5 - a. How does increasing the temperature increase...Ch. 5 - a. Describe activation energy for a chemical...Ch. 5 - Why does the rate of a chemical reaction decrease...Ch. 5 - Prob. 5.14PPCh. 5 - Enzymes increase the rate of a biological chemical...Ch. 5 - Prob. 5.16PPCh. 5 - Prob. 5.17PPCh. 5 - Prob. 5.18PPCh. 5 - Categorize the following reactions as synthesis,...Ch. 5 - Categorize the following reactions as synthesis,...Ch. 5 - Prob. 5.21PPCh. 5 - Prob. 5.22PPCh. 5 - Write the products and balance the following...Ch. 5 - Prob. 5.24PPCh. 5 - Prob. 5.25PPCh. 5 - List the differences between general chemical...Ch. 5 - Are the substances shown in italics undergoing...Ch. 5 - Prob. 5.28PPCh. 5 - Prob. 5.29PPCh. 5 - Prob. 5.30PPCh. 5 - Prob. 5.31PPCh. 5 - Prob. 5.32PPCh. 5 - Prob. 5.33PPCh. 5 - Prob. 5.34PPCh. 5 - Prob. 5.35PPCh. 5 - Prob. 5.36PPCh. 5 - Prob. 5.37PPCh. 5 - Write the main product of hydration for the...Ch. 5 - Methane (a.k.a. natural gas) can react with oxygen...Ch. 5 - Prob. 5.40APCh. 5 - Which reaction occurs at a faster rate, an...Ch. 5 - Prob. 5.42APCh. 5 - Prob. 5.43APCh. 5 - Two curves for the same reaction are shown in the...Ch. 5 - Prob. 5.45APCh. 5 - Draw and label a reaction energy diagram for an...Ch. 5 - Prob. 5.47APCh. 5 - Prob. 5.48APCh. 5 - Write the products that would result from the...Ch. 5 - Prob. 5.50APCh. 5 - Prob. 5.51APCh. 5 - Prob. 5.52APCh. 5 - Prob. 5.53APCh. 5 - Prob. 5.54APCh. 5 - Prob. 5.55APCh. 5 - Identify the reactant that is oxidized and the...Ch. 5 - Prob. 5.57APCh. 5 - Write the products of the following reactions:Ch. 5 - Prob. 5.59APCh. 5 - Acetylsalicylic acid (aspirin) can be synthesized...Ch. 5 - Prob. 5.61APCh. 5 - Fill in the missing organic produce for the...Ch. 5 - Prob. 5.63APCh. 5 - Prob. 5.64APCh. 5 - How do low-carb diets work? We store glucose...Ch. 5 - Prob. 5.66CPCh. 5 - Prob. 5.67CPCh. 5 - Prob. 5.68CPCh. 5 - Prob. 5.69CPCh. 5 - Which reaction has the larger activation energy?Ch. 5 - Prob. 1IA.2QCh. 5 - A catalyst speeds up a chemical reaction by...Ch. 5 - Prob. 1IA.4QCh. 5 - Examine your sketch from question 3. Does a...Ch. 5 - Prob. 1IA.6QCh. 5 - Prob. 2IA.1QCh. 5 - Prob. 2IA.2QCh. 5 - Prob. 2IA.3QCh. 5 - One of the reactions in the data set is a single...Ch. 5 - Prob. 2IA.5QCh. 5 - Categorize the following reactions as a synthesis,...Ch. 5 - Prob. 3IA.1QCh. 5 - Prob. 3IA.2QCh. 5 - Prob. 3IA.3QCh. 5 - Prob. 3IA.4QCh. 5 - Prob. 3IA.5QCh. 5 - Prob. 1ICCh. 5 - Find out how unsaturated fats are saturated and...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If the current voltage is n = 0.14 V. Indicate which of the 2 formulas must be applied a) = a T = i exp[(1 - p) F Fn Fn b) i==exp B RTarrow_forwardTopic: Photochemistry and Photophysics of Supramoleculesarrow_forwardTwo cations that exchange an electron in an interface, the exchange density is worth 1.39 mA/cm2 and the current density is worth 15 mA/cm2 at 25°C. If the overvoltage is 0.14 V, calculate the reaction rate and symmetry factor. Data: R = 8,314 J mol-1 k-1: F = 96500 Carrow_forward
- With the help of the Tafel line, it is estimated that the interchange density of the VO2+/VO2+ system on the carbon paper has a value of 3 mA cm-2. Calculate a) the current density if the voltage has a value of 1.6 mV and the temperature is 25°C. b) the beta value of the anódico process if the Tafel pendulum is 0.6 V at 25°C. Data: R = 8.314 JK-1mol-1, y F = 96485 C mol-1.arrow_forwardApply the NANSTE law to the MnO4- + 8H+ + 5e- ⇄ Mn2+ + 4H2Oarrow_forwardIn the Nernst Law, how much is RT / F?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div
Calorimetry Concept, Examples and Thermochemistry | How to Pass Chemistry; Author: Melissa Maribel;https://www.youtube.com/watch?v=nSh29lUGj00;License: Standard YouTube License, CC-BY