
Concept explainers
(a)
Interpretation:
Considering a gas container equipped with a movable piston, the change in volume and pressure should be explained.
Concept Introduction:
Ideal gas equation:
At a constant temperature (K) and pressure (P), the volume (v) occupied by the no of moles of any gas is known as ideal gas equation.
Ideal gas equation:
And the SI units are
(a)
Answer to Problem 5.31QP
An increase of pressure by 2 times to the original, will decrease volume by
Explanation of Solution
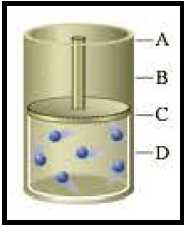
Figure 1
From
Pressure and volume are inversely proportional to each other. So the increase in pressure causes decrease in volume C to D.
The change in volume with respect to pressure was explained.
(b)
Interpretation:
Considering a gas container equipped with a movable piston, the change in volume and pressure should be explained.
Concept Introduction:
Ideal gas equation:
At a constant temperature (K) and pressure (P), the volume (v) occupied by the no of moles of any gas is known as ideal gas equation.
Ideal gas equation:
And the SI units are
(b)
Answer to Problem 5.31QP
An increase of volume (C to A) by 2 times to the original, will decrease pressure by
Explanation of Solution

Figure 1
From Ideal
Pressure and volume are inversely proportional to each other. So the increase in volume (C to A) causes decrease in pressure as molecules get more space to move around.
The change in volume with respect to pressure was explained.
(c)
Interpretation:
Considering a gas container equipped with a movable piston, the change in temperature when volume changes from C to B should be explained.
Concept Introduction:
Ideal gas equation:
At a constant temperature (K) and pressure (P), the volume (v) occupied by the no of moles of any gas is known as ideal gas equation.
Ideal gas equation:
And the SI units are
(c)
Answer to Problem 5.31QP
The change in kelvin temperature by 1.5 factor would cause the change in volume (C to B)
Explanation of Solution

Figure 1
From Ideal gas law,
Temperature and volume are proportional to each other. So the increase in volume (C to A) causes increase in temperature.
The change in volume with respect to temperature was explained
(d)
Interpretation:
The change in volume and pressure when the number of moles increased in a container with a moving piston should be explained.
Concept Introduction:
Ideal gas equation:
At a constant temperature (K) and pressure (P), the volume (v) occupied by the no of moles of any gas is known as ideal gas equation.
Ideal gas equation:
And the SI units are
(d)
Answer to Problem 5.31QP
The increase in the number of moles by 2 factor would cause the increase in volume by 2 factor (C to A).
Explanation of Solution

Figure 1
From Ideal gas law,
Number of particles and volume are proportional to each other. So the increase in molecules would increase the volume by 2 factor (C to A). Since the piston is movable the pressure will not be affected (similar to starting pressure) even though number of particles and pressure are directly proportional to each other.
The change in volume and pressure when the number of moles increased in a container with a moving piston was explained.
Want to see more full solutions like this?
Chapter 5 Solutions
Bundle: General Chemistry, Loose-leaf Version, 11th + OWLv2, 4 terms (24 months) Printed Access Card
- curved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the cured electron-pushing arrows for thw following reaction or mechanistic steps. be sure to account for all bond-breaking and bond making stepsarrow_forwardUsing the graphs could you help me explain the answers. I assumed that both graphs are proportional to the inverse of time, I think. Could you please help me.arrow_forwardSynthesis of Dibenzalacetone [References] Draw structures for the carbonyl electrophile and enolate nucleophile that react to give the enone below. Question 1 1 pt Question 2 1 pt Question 3 1 pt H Question 4 1 pt Question 5 1 pt Question 6 1 pt Question 7 1pt Question 8 1 pt Progress: 7/8 items Que Feb 24 at You do not have to consider stereochemistry. . Draw the enolate ion in its carbanion form. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. ⚫ Separate multiple reactants using the + sign from the drop-down menu. ? 4arrow_forward
- Shown below is the mechanism presented for the formation of biasplatin in reference 1 from the Background and Experiment document. The amounts used of each reactant are shown. Either draw or describe a better alternative to this mechanism. (Note that the first step represents two steps combined and the proton loss is not even shown; fixing these is not the desired improvement.) (Hints: The first step is correct, the second step is not; and the amount of the anhydride is in large excess to serve a purpose.)arrow_forwardHi I need help on the question provided in the image.arrow_forwardDraw a reasonable mechanism for the following reaction:arrow_forward
- Draw the mechanism for the following reaction: CH3 CH3 Et-OH Et Edit the reaction by drawing all steps in the appropriate boxes and connecting them with reaction arrows. Add charges where needed. Electron-flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. H± EXP. L CONT. י Α [1] осн CH3 а CH3 :Ö Et H 0 N о S 0 Br Et-ÖH | P LL Farrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward20.00 mL of 0.025 M HCl is titrated with 0.035 M KOH. What volume of KOH is needed?arrow_forward
- 20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward20.00 mL of 0.025 M HCl is titrated with 0.035 M KOH. What volume of KOH is needed?arrow_forward20.00 mL of 0.150 M HCl is titrated with 37.75 mL of NaOH. What is the molarity of the NaOH?arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





