
Chemistry, Books a la Carte Plus Mastering Chemistry with eText -- Access Card Package (7th Edition)
7th Edition
ISBN: 9780133900811
Author: John E. McMurry, Robert C. Fay, Jill Kirsten Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 5.27CP
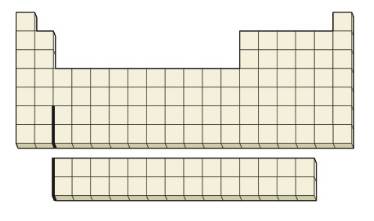
Where on the blank outline of the periodic table do elements that meet the following descriptions appear?
(a) Elements with the valence-shell ground-state electron con figuration ns2np5
(b) An element with the ground-state electron configuration [Ar] 4s23d104p5
(c) Elements with electrons whose largest principal quantum number is n = 4
(d) Elements that have only one unpaired p electron
(e) The d-block elements
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't used hand raiting
Don't used hand raiting
Don't used hand raiting
Chapter 5 Solutions
Chemistry, Books a la Carte Plus Mastering Chemistry with eText -- Access Card Package (7th Edition)
Ch. 5 - Prob. 5.1PCh. 5 - Conceptual APPLY 5.2 Two electromagnetic waves are...Ch. 5 - PRACTICE 5.3 The biological effects of a given...Ch. 5 - Prob. 5.4ACh. 5 - Prob. 5.5PCh. 5 - Conceptual APPLY 5.6 Compare the two elements Rb...Ch. 5 - PRACTICE 5.7 The Balmer equation can be extended...Ch. 5 - APPLY 5.8 (a) What is the longest-wavelength line...Ch. 5 - Prob. 5.9PCh. 5 - Prob. 5.10A
Ch. 5 - Prob. 5.11PCh. 5 - APPLY 5.10 Extend Table 5.2 to show allowed...Ch. 5 - Conceptual PRACTICE 5.13 Give a possible...Ch. 5 - Conceptual APPLY 5.14 How many nodal planes...Ch. 5 - Prob. 5.15PCh. 5 - Conceptual APPLY 5.16 Identify the atoms with the...Ch. 5 - Prob. 5.17PCh. 5 - APPLY 5.18 Predict which bond length will be the...Ch. 5 - PROBLEM 5.19 Mercury vapor is contained inside the...Ch. 5 - PROBLEM 5.20 When electricity is used to add...Ch. 5 - Prob. 5.21PCh. 5 - PROBLEM 5.22 Three different wavelengths in the...Ch. 5 - Prob. 5.23PCh. 5 - Two electromagnetic waves are represented below....Ch. 5 - The following diagram shows the energy levels of...Ch. 5 - Identify each of the following orbitals, and give...Ch. 5 - Where on the blank outline of the periodic table...Ch. 5 - One of the elements shown on the following...Ch. 5 - What atom has the following orbital-filling...Ch. 5 - The following orbital-filling diagram represents...Ch. 5 - Prob. 5.31CPCh. 5 - Which has the higher frequency, red light or...Ch. 5 - Prob. 5.33SPCh. 5 - The Hubble Space Telescope detects electromagnetic...Ch. 5 - Prob. 5.35SPCh. 5 - Prob. 5.36SPCh. 5 - Prob. 5.37SPCh. 5 - Prob. 5.38SPCh. 5 - Prob. 5.39SPCh. 5 - Prob. 5.40SPCh. 5 - Prob. 5.41SPCh. 5 - Prob. 5.42SPCh. 5 - Prob. 5.43SPCh. 5 - Prob. 5.44SPCh. 5 - Prob. 5.45SPCh. 5 - Prob. 5.46SPCh. 5 - Prob. 5.47SPCh. 5 - Prob. 5.48SPCh. 5 - Prob. 5.49SPCh. 5 - Prob. 5.50SPCh. 5 - Prob. 5.51SPCh. 5 - Prob. 5.52SPCh. 5 - Prob. 5.53SPCh. 5 - Prob. 5.54SPCh. 5 - Prob. 5.55SPCh. 5 - Spectroscopy is a technique that uses the...Ch. 5 - Prob. 5.57SPCh. 5 - Prob. 5.58SPCh. 5 - Prob. 5.59SPCh. 5 - Prob. 5.60SPCh. 5 - Prob. 5.61SPCh. 5 - Prob. 5.62SPCh. 5 - What velocity would an electron ( mass=9.111031...Ch. 5 - Prob. 5.64SPCh. 5 - Prob. 5.65SPCh. 5 - Prob. 5.66SPCh. 5 - Prob. 5.67SPCh. 5 - Prob. 5.68SPCh. 5 - Prob. 5.69SPCh. 5 - Prob. 5.70SPCh. 5 - Prob. 5.71SPCh. 5 - Prob. 5.72SPCh. 5 - Prob. 5.73SPCh. 5 - Prob. 5.74SPCh. 5 - Prob. 5.75SPCh. 5 - Prob. 5.76SPCh. 5 - Prob. 5.77SPCh. 5 - Prob. 5.78SPCh. 5 - Prob. 5.79SPCh. 5 - Prob. 5.80SPCh. 5 - Prob. 5.81SPCh. 5 - Prob. 5.82SPCh. 5 - Prob. 5.83SPCh. 5 - Prob. 5.84SPCh. 5 - Prob. 5.85SPCh. 5 - Prob. 5.86SPCh. 5 - Prob. 5.87SPCh. 5 - Prob. 5.88SPCh. 5 - Prob. 5.89SPCh. 5 - Prob. 5.90SPCh. 5 - Prob. 5.91SPCh. 5 - Prob. 5.92SPCh. 5 - Prob. 5.93SPCh. 5 - Prob. 5.94SPCh. 5 - Prob. 5.95SPCh. 5 - Prob. 5.96SPCh. 5 - Draw orbital-filling diagrams for atoms with the...Ch. 5 - Prob. 5.98SPCh. 5 - Prob. 5.99SPCh. 5 - Prob. 5.100SPCh. 5 - Prob. 5.101SPCh. 5 - Prob. 5.102SPCh. 5 - Prob. 5.103SPCh. 5 - Prob. 5.104SPCh. 5 - Prob. 5.105SPCh. 5 - Prob. 5.106SPCh. 5 - Prob. 5.107SPCh. 5 - Prob. 5.108SPCh. 5 - Prob. 5.109SPCh. 5 - What is the expected ground-state electron...Ch. 5 - What is the atomic number and expected...Ch. 5 - Prob. 5.112CPCh. 5 - Prob. 5.113CPCh. 5 - Prob. 5.114CPCh. 5 - 5.115 Lines in a certain series of the hydrogen...Ch. 5 - Prob. 5.116CPCh. 5 - Prob. 5.117CPCh. 5 - Prob. 5.118CPCh. 5 - Prob. 5.119CPCh. 5 - Prob. 5.120CPCh. 5 - Prob. 5.121CPCh. 5 - Prob. 5.122CPCh. 5 - Prob. 5.123CPCh. 5 - Prob. 5.124CPCh. 5 - Prob. 5.125CPCh. 5 - Prob. 5.126CPCh. 5 - Prob. 5.127CPCh. 5 - Prob. 5.128CPCh. 5 - Prob. 5.129CPCh. 5 - Prob. 5.130CPCh. 5 - Prob. 5.131CPCh. 5 - Prob. 5.132CPCh. 5 - Prob. 5.133CPCh. 5 - Prob. 5.134CPCh. 5 - Prob. 5.135CPCh. 5 - Prob. 5.136CPCh. 5 - Prob. 5.137CPCh. 5 - Prob. 5.138CPCh. 5 - Prob. 5.139MPCh. 5 - Prob. 5.140MPCh. 5 - Prob. 5.141MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can you please explain why the correct answer for this question is letter B? I chose letter A because I thought that a kinetic product was a 1,2-addition. Please give a detailed explanation.arrow_forwardCan you please explain why the answer is structures 2 and 3? Please include a detailed explanation and show how the synthesis can be done with those two structures.arrow_forwardCan you please explain why the correct answer to this question is option 2? I am having trouble understanding how and why. Please provide a detailed explanation and a drawing of how the diene and dienophile would create the product in the question.arrow_forward
- Can you please explain why the correct answer is molecules 2 and 4? Base your explanation off of the rules for aromaticity and well as the principles of the Huckel rule of aromaticity. Please give a detailed explanation of what Hucekl's rule is.arrow_forwardCan you please explain why the answer is B and not A? I chose A because I thought the thermodynamic product was a 1,4-addition. Please give a detailed explanation to this problem and include a drawing of how the reaction works.arrow_forwardLabel the diagram according to the components and processes of an alkaline batteryarrow_forward
- Can you please explain why the answer to the question is option 4? Please include the aromaticity rules as well as Huckel's rule. Please label molecules 1, 2, 3, and 5 with their respective labels of aromatic or nonaromatic and why.arrow_forwardDon't used hand raitingarrow_forwardCan you please explain why the correct answer is molecules 2 and 4? Please provide a detailed explanation as well as the two molecules drawn showing what and where it is conjugated.arrow_forward
- Can you please explain why the correct answer is (2E, 4Z, 6Z)-2,4,6-Nonatriene? Please include a detailed explanation and a drawing of the structure, with the corresponding parts of the answer labeled. I'm confused why 6 is Z and why it is Nonatriene.arrow_forward? /1600 O Macmillan Learning Using the data in the table, determine the rate constant of the Trial [A] (M) [B] (M) Rate (M/s) reaction and select the appropriate units. 1 0.240 0.350 0.0187 2 0.240 0.700 0.0187 A+2B C+D 3 0.480 0.350 0.0748 k = Unitsarrow_forwardCan you please explain why structure 3 is the correct answer? I am having trouble understanding why it is aromatic. Can you also label molecules 1, 2, 4, and 5 with the correct nonaromatic or antiaromatic?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax
The Bohr Model of the atom and Atomic Emission Spectra: Atomic Structure tutorial | Crash Chemistry; Author: Crash Chemistry Academy;https://www.youtube.com/watch?v=apuWi_Fbtys;License: Standard YouTube License, CC-BY