
Concept explainers
(a)
Interpretation:
Atomic orbitals which are used to form each
Concept Introduction:
Hybridization is the mixing of valence atomic orbitals to get equivalent hybridized orbitals that having similar characteristics and energy.
Sigma (σ) bonds are the bonds in which shared hybrid orbital’s electron density are concentrated along the internuclear axis.
Pi (π) bonds are the bonds in which shared unhybridized orbital’s (p, d, etc) electron density are concentrated in above and below of the plane of the molecule.
Geometry of different types of molecule with respect to the hybridizations are mentioned are mentioned below,
(a)
Explanation of Solution
In the marked carbon atom, one s and three p orbital hybridize forming four
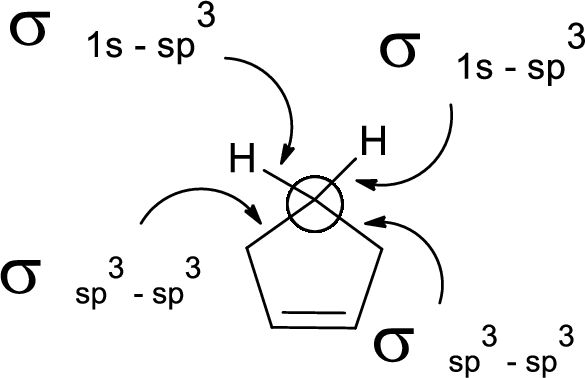
In the marked carbon atom, one s and two p orbital hybridize forming three
(b)
Interpretation:
Atomic orbitals which are used to form each
Concept Introduction:
Hybridization is the mixing of valence atomic orbitals to get equivalent hybridized orbitals that having similar characteristics and energy.
Sigma (σ) bonds are the bonds in which shared hybrid orbital’s electron density are concentrated along the internuclear axis.
Pi (π) bonds are the bonds in which shared unhybridized orbital’s (p, d, etc) electron density are concentrated in above and below of the plane of the molecule.
Geometry of different types of molecule with respect to the hybridizations are mentioned are mentioned below,
(b)
Explanation of Solution
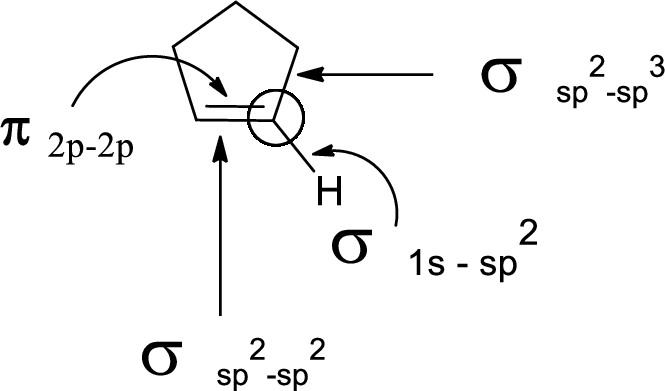
In the marked carbon atom, one s and two p orbital hybridize forming three
(c)
Interpretation:
Atomic orbitals which are used to form each
Concept Introduction:
Hybridization is the mixing of valence atomic orbitals to get equivalent hybridized orbitals that having similar characteristics and energy.
Sigma (σ) bonds are the bonds in which shared hybrid orbital’s electron density are concentrated along the internuclear axis.
Pi (π) bonds are the bonds in which shared unhybridized orbital’s (p, d, etc) electron density are concentrated in above and below of the plane of the molecule.
Geometry of different types of molecule with respect to the hybridizations are mentioned are mentioned below,
(c)
Explanation of Solution
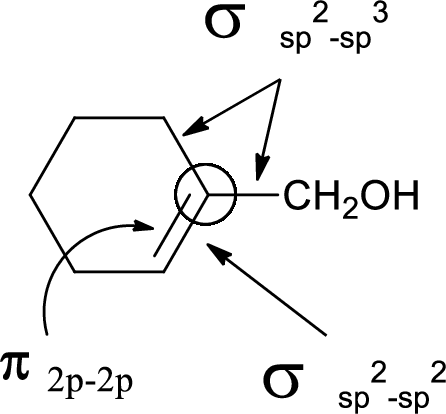
In the marked carbon atom, one s and two p orbital hybridize forming three
(d)
Interpretation:
Atomic orbitals which are used to form each
Concept Introduction:
Hybridization is the mixing of valence atomic orbitals to get equivalent hybridized orbitals that having similar characteristics and energy.
Sigma (σ) bonds are the bonds in which shared hybrid orbital’s electron density are concentrated along the internuclear axis.
Pi (π) bonds are the bonds in which shared unhybridized orbital’s (p, d, etc) electron density are concentrated in above and below of the plane of the molecule.
Geometry of different types of molecule with respect to the hybridizations are mentioned are mentioned below,
(d)
Explanation of Solution
In the marked carbon atom, one s and two p orbital hybridize forming three
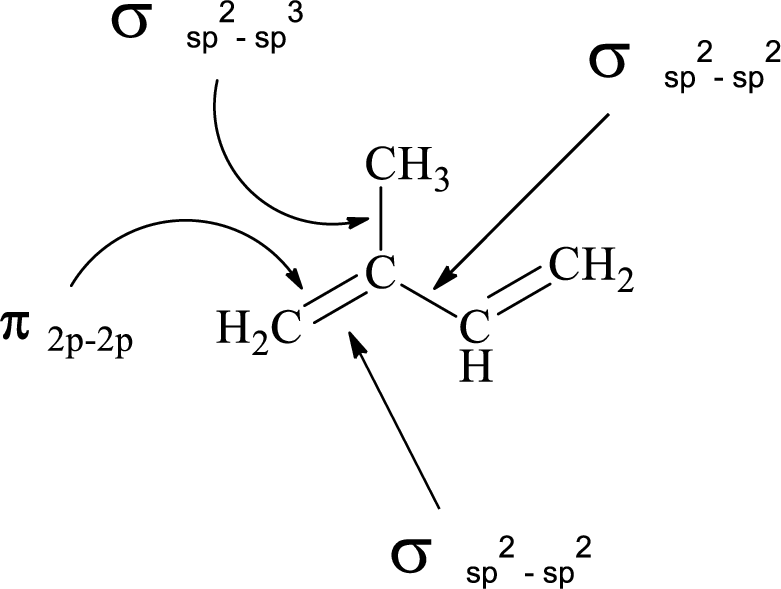
(e)
Interpretation:
Atomic orbitals which are used to form each
Concept Introduction:
Hybridization is the mixing of valence atomic orbitals to get equivalent hybridized orbitals that having similar characteristics and energy.
Sigma (σ) bonds are the bonds in which shared hybrid orbital’s electron density are concentrated along the internuclear axis.
Pi (π) bonds are the bonds in which shared unhybridized orbital’s (p, d, etc) electron density are concentrated in above and below of the plane of the molecule.
Geometry of different types of molecule with respect to the hybridizations are mentioned are mentioned below,
(e)
Explanation of Solution
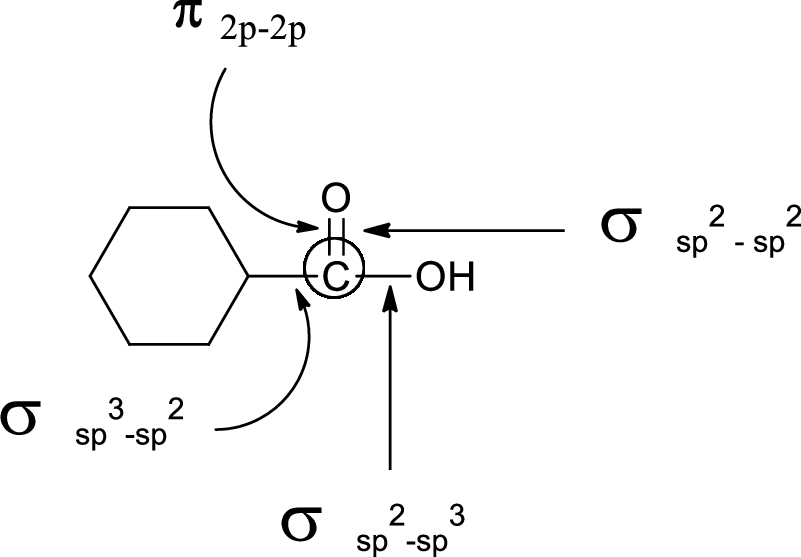
In the marked carbon atom, one s and one p orbital hybridize forming two
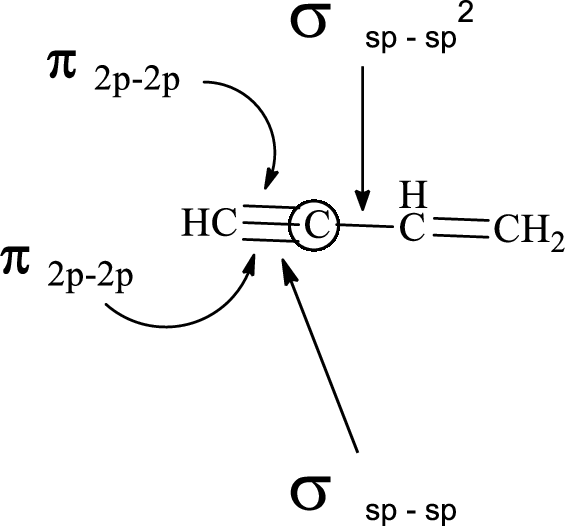
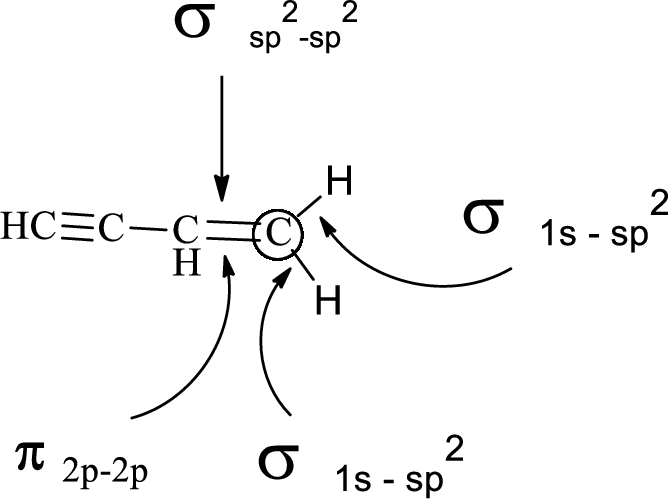
In the marked carbon atom, one s and two p orbital hybridize forming three
Want to see more full solutions like this?
Chapter 5 Solutions
ORGANIC CHEMISTRY-OWL V2 ACCESS
- Write the systematic name of each organic molecule: structure HO-C-CH2-CH3 O -OH CH3-CH2-CH2-CH2-CH2-C-OH CH3 CH3-CH-CH2-C-OH Explanation Check S namearrow_forwardtheres 2 productsarrow_forwardDraw the major product of this solvolysis reaction. Ignore any inorganic byproducts. + CH3CH2OH Drawing Q Atoms, Bonds and Rings OCH2CH3 || OEt Charges OH 00-> | Undo Reset | Br Remove Done Drag To Pan +arrow_forward
- Draw the major product of this SN1 reaction. Ignore any inorganic byproducts. CH3CO2Na CH3CO2H Drawing + Br Q Atoms, Bonds and Rings OAC Charges OH ОАс Na ဂ Br Undo Reset Remove Done Drag To Pan +arrow_forwardOrganic Functional Groups entifying positions labeled with Greek letters in acids and derivatives 1/5 ssible, replace an H atom on the a carbon of the molecule in the drawing area with a ce an H atom on the ẞ carbon with a hydroxyl group substituent. ne of the substituents can't be added for any reason, just don't add it. If neither substi er the drawing area. O H OH Oneither substituent can be added. Check D 1 Accessibility ado na witharrow_forwardDifferentiate between electrophilic and nucleophilic groups. Give examples.arrow_forward
- An aldehyde/ketone plus an alcohol gives a hemiacetal, and an excess of alcohol gives an acetal. The reaction is an equilibrium; in aldehydes, it's shifted to the right and in ketones, to the left. Explain.arrow_forwardDraw a Haworth projection or a common cyclic form of this monosaccharide: H- -OH H- OH H- -OH CH₂OHarrow_forwardAnswer the question in the first photoarrow_forward
- Ggggffg2258555426855 please don't use AI Calculate the positions at which the probability of a particle in a one-dimensional box is maximum if the particle is in the fifth energy level and in the eighth energy level.arrow_forwardExplain the concepts of hemiacetal and acetal.arrow_forwardBriefly describe a nucleophilic addition.arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





