
Chemistry for Changing Times
14th Edition
ISBN: 9780134212777
Author: John W. Hill; Terry W. McCreary
Publisher: Pearson Education (US)
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 5, Problem 22P
Interpretation Introduction
Interpretation: The ratio of volume of 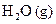 formed to volume of
formed to volume of 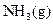 that reacts has to be determined.
that reacts has to be determined.
Concept introduction:
- The volume occupied by 1 mole of an ideal gas is 22.4 L at STP (standard temperature and pressure), standard temperature being
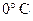 and standard pressure being
and standard pressure being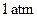
- This volume is called the molar volume and the law which states the above statement is called the
Avogadro’s law .
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For this question, if the product is racemic, input both enantiomers in the same Marvin editor.
A) Input the number that corresponds to the reagent which when added to (E)-but-2-ene will result in a
racemic product.
Input 1 for Cl, in the cold and dark
Input 2 for Oy followed by H₂O, Zn
Input 3 for D₂ with metal catalyst
Input 4 for H₂ with metal catalyst
B) Draw the skeletal structure of the major organic product made from the reagent in part A
Marvin JS
Help
Edit drawing
C) Draw the skeletal structure of the major organic product formed when (2)-but-2-ene is treated with
peroxyacetic acid.
Marvin 35
Help
Michael Reactions
19.52 Draw the products from the following Michael addition reactions.
1.
H&C CH
(a)
i
2. H₂O*
(b)
OEt
(c)
EtO
H₂NEt
(d)
ΕΙΟ
+
1. NaOEt
2. H₂O'
H
H
1. NaOEt
2. H₂O*
Rank the labeled protons (Ha-Hd) in order of increasing acidity, starting with the least acidic.
НОН НЬ
OHd
Онс
Chapter 5 Solutions
Chemistry for Changing Times
Ch. 5 - Prob. 1RQCh. 5 - Prob. 2RQCh. 5 - What is Avogadro's hypothesis? How does it explain...Ch. 5 - Prob. 4RQCh. 5 - 5. Define or explain and illustrate the following...Ch. 5 - Prob. 6RQCh. 5 - Prob. 7PCh. 5 - Prob. 8PCh. 5 - Prob. 9PCh. 5 - Prob. 10P
Ch. 5 - Consider the following equation. (a) Explain its...Ch. 5 - Prob. 12PCh. 5 - Prob. 13PCh. 5 - Prob. 14PCh. 5 - Prob. 15PCh. 5 - Prob. 16PCh. 5 - Prob. 17PCh. 5 - Prob. 18PCh. 5 - Prob. 19PCh. 5 - Prob. 20PCh. 5 - Prob. 21PCh. 5 - Prob. 22PCh. 5 - Prob. 23PCh. 5 - Prob. 24PCh. 5 - Prob. 25PCh. 5 - Prob. 26PCh. 5 - Prob. 27PCh. 5 - Prob. 28PCh. 5 - Prob. 29PCh. 5 - Prob. 30PCh. 5 - Prob. 31PCh. 5 - Prob. 32PCh. 5 - Prob. 33PCh. 5 - Prob. 34PCh. 5 - Prob. 35PCh. 5 - Consider the reaction for the combustion of octane...Ch. 5 - Prob. 37PCh. 5 - Toluene (C7H8) and nitric acid (HNO3) are used in...Ch. 5 - Prob. 39PCh. 5 - Prob. 40PCh. 5 - Prob. 41PCh. 5 - Prob. 42PCh. 5 - Prob. 43PCh. 5 -
44. What volume in liters of (a) 0.250 M NaOH...Ch. 5 - Prob. 45PCh. 5 - Prob. 46PCh. 5 - Prob. 47PCh. 5 - Prob. 48PCh. 5 - Prob. 49PCh. 5 - Prob. 50PCh. 5 - Prob. 51APCh. 5 - Prob. 52APCh. 5 - Prob. 53APCh. 5 - Prob. 54APCh. 5 - Prob. 55APCh. 5 - Prob. 56APCh. 5 - Prob. 57APCh. 5 - Prob. 58APCh. 5 - Prob. 59APCh. 5 - Prob. 60APCh. 5 - Prob. 61APCh. 5 - Prob. 62APCh. 5 - Prob. 63APCh. 5 - Prob. 64APCh. 5 - Prob. 65APCh. 5 - Prob. 66APCh. 5 - Prob. 67APCh. 5 - Evaluate this statement: ‘One cup of water has...Ch. 5 - Prob. 69APCh. 5 - Prob. 70APCh. 5 - Prob. 71APCh. 5 - Prob. 72APCh. 5 - Prob. 73APCh. 5 - Prob. 74APCh. 5 - Prob. 75APCh. 5 - Prob. 5.1CTECh. 5 - Prob. 5.2CTECh. 5 - Prob. 5.3CTECh. 5 - Prob. 5.4CTECh. 5 - Prob. 5.5CTECh. 5 - Prob. 1CGPCh. 5 - Prob. 2CGPCh. 5 - Prob. 1CHQCh. 5 - Prob. 2CHQCh. 5 - Prob. 3CHQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? ? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C :0 T Add/Remove step Garrow_forwardThe following equations represent the formation of compound MX. What is the AH for the electron affinity of X (g)? X₂ (g) → 2X (g) M (s) → M (g) M (g) M (g) + e- AH = 60 kJ/mol AH = 22 kJ/mol X (g) + e-X (g) M* (g) +X (g) → MX (s) AH = 118 kJ/mol AH = ? AH = -190 kJ/mol AH = -100 kJ/mol a) -80 kJ b) -30 kJ c) -20 kJ d) 20 kJ e) 156 kJarrow_forwardA covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forward
- Can the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forwardWhich one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forwardAll of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forward
- A student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forwardPredict the major products of this organic reaction:arrow_forwardCalculate the density of 21.12 g of an object that displaces 0.0250 L of water.arrow_forward
- Draw the expected reactant R28. Cu(II) CO₂Mearrow_forwardPpplllleeeaaasssseeee helllppp wiithhh thisss Organic chemistryyyyyy I talked like this because AI is very annoyingarrow_forwardName the family to which each organic compound belongs. The first answer has been filled in for you. compound CH₂ || CH3-C-NH2 0 ။ CH3-C-CH₂ CH=O–CH=CH, CH₂ HO CH2-CH2-CH-CH3 family amine Darrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY