
Interpretation:
The principle behind the working of barometer and manometer has to be explained.
Concept Introduction:
The force acting per unit area is called as Pressure. Differential pressure is indicated by measuring devices i.e. in relative with the atmosphere pressure. This is known as gauge pressure. The calculated pressure can either be positive or negative with respect to the atmospheric pressure. Vacuum is generally known as a negative gauge pressure.
Atmospheric pressure is measured by using an instruments called as barometer and manometer.
Explanation of Solution
A barometer consists of Mercury column that is tipped inverted and positioned in a dish containing Mercury. The Mercury in the column moves to and fro. The measure of atmospheric pressure is done by the height of the column. There is a downward force produced by the weight of the Mercury, this downward force pushes the mercury to fall out of the column. But, there is repulsive force that keeps Mercury in the column. This repulsive force is due to the atmospheric gas particles that collide with the surface of the Mercury in the dish; this makes the mercury to get pushed up in the column. The level of Mercury in the column stays constant, when the two repulsive forces are same in strength to each other. The Mercury’s constant height is supported by the atmosphere is a measure of pressure of the atmosphere.
A simple barometer is illustrated in the figure 1,
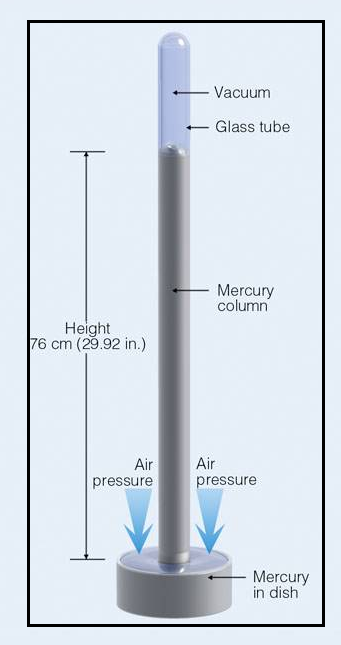
Figure 1: Simple Barometer
To explain the working of Manometer
A manometer also has two repulsive forces against each other. A force is exerted by the gas molecules in the flask. The repulsive force is seen on the further region of the Mercury filled U tube; that is force exerted by atmospheric gases. The measure of the variation in pressure between the gas in the flask and the atmosphere gives the difference in height of the Mercury in the U tube. The greater or lesser the gas in the flask to the atmosphere pressure can be determined by measuring the difference in the height of the Mercury.
Illustration of simple manometer is shown in figure 2,
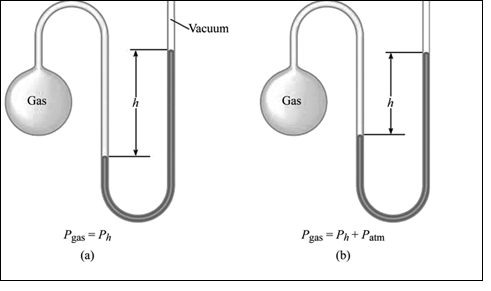
Figure 2: A simple manometer
The principle and working of barometer and manometer are explained.
Want to see more full solutions like this?
Chapter 5 Solutions
CHEMISITRY W/OWL PKG LOOSELEAF
- Select/ Match the correct letter from the image below for the IUPAC names given below: A B C D 3 E F G H K L Part 1. 4-methylheptane For example.mmmm Answer Letter H _for part 1 Part 2. 2,4-dimethylhexane Part 3. 2,3-dimethylpentane Part 4. 2,2-dimethylhexane Part 5. 2-ethyl-1,1,3,3-tetramethylcyclopentane Part 6. 3-ethyl-2-methylpentanearrow_forwardCan u show the process as to how to get these?arrow_forwardSketch the expected 'H NMR spectra for the following compound. Label all of the H's in the structure and the corresponding signal for the spectra you sketch. Make sure you include the integration value and the splitting pattern for each signal Indicate how many signals you would expect in the 13C NMRarrow_forward
- Use IUPAC naming rules to name the following hydrocarbon compounds: CH2-CH3 | a) CH-CH-CH2-CH-CH-CH3 b) | CH2 CH3 | CH3 CH3 \ / C=C H 1 H CH2-CH3 c) d) CH=C-CH3 e) CH3-CH2-CH2-CH=CH-CH3 f) CH2=CH-CH2-CH=CH-CH3 g) CH3-CH2-C = C-CH2-CH3 h)arrow_forwardQ5 Name the following : a. b. C. d. e.arrow_forward25. Predict the major product of the following reaction. 1 equivalent of each of the starting materials was used. H₂C CH3 CH3 H3C H3C H3C. CH2 + H3C. heat CH3 CH H.C. CH3 H.C H.C CH3 CH CH3 CH3 A B C Earrow_forward
- Find chemical structures based on the below information. a) Chemical formula C6H8O Compound is aromatic plus has two 1H NMR peaks that integrated for 3 each that are singlets (it could have more peaks in the 1H NMR b) Chemical Formula: C6H100 Compounds is conjugated 'H NMR has a signal that integrates for 6 and is a doublet IR spectra has a signal at 1730 cm-1arrow_forwardJaslev Propose a synthesis of the following starting from benzene and any other reagents and chemicals. No mechanisms are required. Indicate the condition for each step plus the major product for each step. More than two steps are required. Step 1 Step 2 مہد Brarrow_forwardPart C: The line formula for another branched alkane is shown below. i. In the IUPAC system what is the root or base name of this compound? ii. How many alkyl substituents are attached to the longest chain? iii. Give the IUPAC name for this compound.arrow_forward
- Part D: Draw the Structural Formula for 4-ethyl-2-methylhexane Part E. Draw the Structural Formula for 1-chloro-3,3-diethylpentane (Chloro = Cl)arrow_forwardPart B: The line formula for a branched alkane is shown below. a. What is the molecular formula of this compound? Number of C. Number of H b. How many carbon atoms are in the longest chain? c. How many alkyl substituents are attached to this chain?arrow_forward24. What is the major product for the following reaction? Mg J. H.C CH H,C- Then H₂O OH Br C HO E HO H.C CH H.C- CH₂ CH₂ All of these are possiblearrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





