
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
9th Edition
ISBN: 9781305671874
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4.SE, Problem 62AP
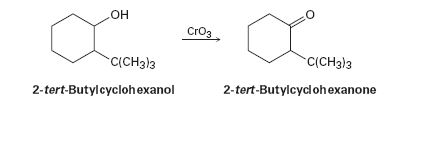
Alcohols undergo an oxidation reaction to yield carbonyl compounds on treatment with CrO3. For example, 2-tert-butylcyclohexanol gives 2-tert-butylcyclohexanone. If axial –OH groups are generally more reactive than their equatorial isomers, which do you think reacts faster, the cis isomer of 2-tert-butylcyclohexanol or the trans isomer? Explain.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
am os
Д
Br
Please use the information from the spectrum to answer these questions
Don't used hand raiting
Chapter 4 Solutions
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
Ch. 4.1 - Give IUPAC names for the following cycloalkanes:Ch. 4.1 - Draw structures corresponding to the following...Ch. 4.1 - Name the following cycloalkane:Ch. 4.2 - Prob. 4PCh. 4.2 - Draw the structures of the following molecules:...Ch. 4.2 - Prostaglandin F2α, a hormone that causes uterine...Ch. 4.2 - Name the following substances, including the cis-...Ch. 4.3 - Each H↔H eclipsing interaction in ethane costs...Ch. 4.3 - cis-1, 2-Dimethylcyclopropane has more strain than...Ch. 4.4 - Prob. 10P
Ch. 4.4 - Two conformations of cis-l, 3-dimethylcyclobutane...Ch. 4.6 - Draw two different chair conformations of...Ch. 4.6 - Draw two differant chair conformations of trans-1,...Ch. 4.6 - Prob. 14PCh. 4.7 - What is the energy difference between the axial...Ch. 4.7 - Prob. 16PCh. 4.7 - Look at Figure 4-12 on page 105, and estimate the...Ch. 4.8 - Draw the more stable chair conformation of the...Ch. 4.8 - Identify each substituent in the following...Ch. 4.9 - Which isomer is more stable, cis-decalin or...Ch. 4.9 - Look at the following structure of the female...Ch. 4.SE - Prob. 22VCCh. 4.SE - Name the following compound, identify each...Ch. 4.SE - A trisubstituted cyclohexane with three...Ch. 4.SE - The following cyclohexane derivative has three...Ch. 4.SE - Prob. 26VCCh. 4.SE - Draw the five cycloalkanes with the formula C5H10.Ch. 4.SE - Draw two constitutional isomers of cis-1,...Ch. 4.SE - Prob. 29APCh. 4.SE - Tell whether the following pairs of compounds are...Ch. 4.SE - Prob. 31APCh. 4.SE - Prob. 32APCh. 4.SE - Draw 1, 3, 5-trimethylcyclohexane using a hexagon...Ch. 4.SE - Hydrocortisone, a naturally occurring hormone...Ch. 4.SE - A 1, 2-cis disubstituted cyclohexane, such as...Ch. 4.SE - A 1, 2-trans disubstituted cyclohexane must have...Ch. 4.SE - Prob. 37APCh. 4.SE - Which is more stable, a 1, 4-trans disubstituted...Ch. 4.SE - cis-1, 2-Dimethylcyclobutane is less stable than...Ch. 4.SE - From the data in Figure 4-12 and Table 4-1,...Ch. 4.SE - Prob. 41APCh. 4.SE - Draw the two chair conformations of...Ch. 4.SE - Draw the two chair conformations of...Ch. 4.SE - Galactose, a sugar related to glucose, contains a...Ch. 4.SE - There are four cis-trans isomers of menthol...Ch. 4.SE - There are four cis-trans isomers of menthol...Ch. 4.SE - The diaxial conformation of cis-1,...Ch. 4.SE - Approximately how much steric strain does the...Ch. 4.SE - In light of your answer to Problem 4-43, draw the...Ch. 4.SE - Prob. 50APCh. 4.SE - Prob. 51APCh. 4.SE - Using molecular models as well as structural...Ch. 4.SE - trans-Decalin is more stable than its cis isomer,...Ch. 4.SE - As mentioned in Problem 3-53, the statin drugs,...Ch. 4.SE - myo-Inositol, one of the isomers of...Ch. 4.SE - How many cis–trans stereoisomers of myo-inositol...Ch. 4.SE - The German chemist J. Bredt proposed in 1935 that...Ch. 4.SE - Tell whether each of the following substituents on...Ch. 4.SE - Prob. 59APCh. 4.SE - Prob. 60APCh. 4.SE - Ketones react with alcohols to yield products...Ch. 4.SE - Alcohols undergo an oxidation reaction to yield...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- X Draw the major products of the elimination reaction below. If elimination would not occur at a significant rate, check the box under the drawing area instead. ది www. Cl + OH Elimination will not occur at a significant rate. Click and drag to start drawing a structure.arrow_forwardNonearrow_forward1A H 2A Li Be Use the References to access important values if needed for this question. 8A 3A 4A 5A 6A 7A He B C N O F Ne Na Mg 3B 4B 5B 6B 7B 8B-1B 2B Al Si P 1B 2B Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe * Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Rf Ha ****** Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Analyze the following reaction by looking at the electron configurations given below each box. Put a number and a symbol in each box to show the number and kind of the corresponding atom or ion. Use the smallest integers possible. cation anion + + Shell 1: 2 Shell 2: 8 Shell 3: 1 Shell 1 : 2 Shell 2 : 6 Shell 1 : 2 Shell 2: 8 Shell 1: 2 Shell 2: 8arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License