
ORGANIC CHEMISTRY W/ALEKS
6th Edition
ISBN: 9781264905430
Author: SMITH
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 4.8, Problem 13P
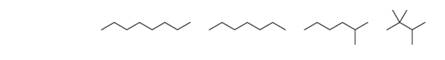
Arrange the following compounds in order of increasing boiling point.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Is this aromatic?
CHEM2323
E
Tt
PS CH03
Draw and name all monobromo derivatives of pentane, C5H11Br.
Problem 3-33
Name:
Draw structures for the following:
(a) 2-Methylheptane
(d) 2,4,4-Trimethylheptane
Problem 3-35
(b) 4-Ethyl-2,2-dimethylhexane
(e) 3,3-Diethyl-2,5-dimethylnonane
(c) 4-Ethyl-3,4-dimethyloctane
2
(f) 4-Isopropyl-3-methylheptane
KNIE>
Problem 3-42
Consider 2-methylbutane (isopentane). Sighting along the C2-C3 bond:
(a) Draw a Newman projection of the most stable
conformation.
(b) Draw a Newman projection of the least stable
conformation.
Problem 3-44
Construct a qualitative potential-energy diagram for rotation about the C-C bond of 1,2-dibromoethane.
Which conformation would you expect to be most stable? Label the anti and gauche conformations of 1,2-
dibromoethane.
Problem 3-45
Which conformation of 1,2-dibromoethane (Problem 3-44) would you expect to have the largest dipole
moment? The observed dipole moment of 1,2-dibromoethane is µ = 1.0 D. What does this tell you about the
actual conformation of the molecule?
Chapter 4 Solutions
ORGANIC CHEMISTRY W/ALEKS
Ch. 4.1 - Prob. 1PCh. 4.1 - Problem 4.2 Which of the following is not another...Ch. 4.1 - Problem 4.3 Draw the five constitutional isomers...Ch. 4.1 - Prob. 4PCh. 4.1 - Prob. 5PCh. 4.2 - Draw the five constitutional isomers that have...Ch. 4.4 - Problem 4.7 Give the IUPAC name for each...Ch. 4.4 - Give the IUPAC name for each compound. a....Ch. 4.4 - Problem 4.9 Give the structure corresponding to...Ch. 4.4 - Prob. 10P
Ch. 4.5 - Give the IUPAC name for each compound.Ch. 4.5 - Give the structure corresponding to each IUPAC...Ch. 4.8 - Arrange the following compounds in order of...Ch. 4.9 - Problem 4.14 Draw the staggered and eclipsed...Ch. 4.9 - Prob. 15PCh. 4.9 - Prob. 16PCh. 4.10 - Problem 4.17 a. Draw the three staggered and...Ch. 4.10 - Problem 4.18 Rank the following conformations in...Ch. 4.10 - Problem 4.19 Consider rotation around the...Ch. 4.10 - Calculate the destabilization present in each...Ch. 4.12 - Problem 4.21 Classify the ring carbons as up or...Ch. 4.12 - Problem 4.22 Using the cyclohexane with the C’s...Ch. 4.13 - Draw a second chair conformation for each...Ch. 4.13 - Problem 4.24 Draw both conformations for and...Ch. 4.13 - Problem 4.25 Draw the structure for each compound...Ch. 4.13 - Prob. 26PCh. 4.14 - Prob. 31PCh. 4.14 - Prob. 32PCh. 4.15 - Prob. 33PCh. 4 - Name each alkane using the ball-and-stick model,...Ch. 4 -
4.40 Draw the structure corresponding to each...Ch. 4 - 4.42 Give the IUPAC name for each compound.
a....Ch. 4 - Prob. 42PCh. 4 - 4.46 Considering rotation around the bond...Ch. 4 - 4.50 Calculate the barrier to rotation for each...Ch. 4 - 4.51 The eclipsed conformation of is less...Ch. 4 - (a) Draw the anti and gauche conformations for...Ch. 4 - For each compound drawn below: a.Label each OH,Br...Ch. 4 - Draw the two possible chair conformations for...Ch. 4 - For each compound drawn below: a. Draw...Ch. 4 - Classify each pair of compounds as constitutional...Ch. 4 - Prob. 66PCh. 4 - 4.64 Draw the products of combustion of each...Ch. 4 - 4.65 Hydrocarbons like benzene are metabolized in...Ch. 4 - Prob. 69PCh. 4 - Prob. 70PCh. 4 - Cyclopropane and cyclobutane have similar strain...Ch. 4 - Prob. 72PCh. 4 - Haloethanes (CH3CH2X,X=Cl,Br,I) have similar...Ch. 4 - Prob. 74PCh. 4 - Prob. 75PCh. 4 - Consider the tricyclic structure B (a) Label each...Ch. 4 - Read Appendix B on naming branched alkyl...Ch. 4 - Read Appendix B on naming bicyclic compounds. Then...
Additional Science Textbook Solutions
Find more solutions based on key concepts
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
To test your knowledge, discuss the following topics with a study partner or in writing ideally from memory. Th...
HUMAN ANATOMY
56. Global Positioning System. Learn more about the global positioning system and its uses. Write a short repo...
The Cosmic Perspective (8th Edition)
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Describe the role and impact of microbes on the earth.
Microbiology Fundamentals: A Clinical Approach
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Gas Law Studies 1. Mass of zinc Determination of 0.899 2) Moles of zinc 0.01361 mol 3.) Moles of hydrogen 00? ← I was told to calculate this number from mole of zinc. 350m So does that mean it will be 0.01361 mol too? 4 Volume of water collected (mL) 5) VL of water collected (Liters) 0.350 L 6) Temp of water collected (°C) 7) Temp of water collected (°K) 8) Atmospheric pressure (mm) 9) Vapor pressure of water (mm) 10) Corrected pressure of hydrogen 20% 29°C 764.0mm Hg (mm) 17.5mm 11) Corrected pressure of hydrogen (atm) 12) Experimentally calculated value of 19 13. Literature value of R 14) % Error 15) Suggest reasons for the % error (#14)arrow_forwardNo wedge or dashes. Do proper structure. Provide steps and explanation.arrow_forward10 Question (1 point) Draw curved arrow notation to indicate the proton transfer between NaOH and CH3CO₂H. 2nd attempt :0- H See Periodic Table See Hint Draw the products of the proton transfer reaction. Don't add a + sign between the products.arrow_forward
- Nonearrow_forward4. Experimental Procedure. a. How many (total) data plots are to be completed for this experiment? Account for each. b. What information is to be extracted from each data plot?arrow_forwardProvide the IUPAC name of the following molecule. Don't forget to include the proper stereochemistry where appropriate.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY